01973
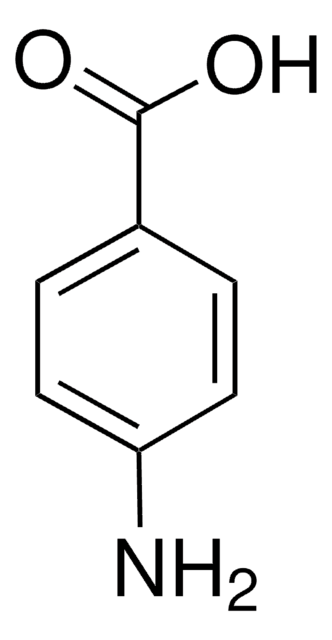
4-Aminobenzoic acid
analytical standard
Synonym(s):
PABA, Vitamin Bx, Vitamin H1
About This Item
Recommended Products
grade
analytical standard
Quality Level
Assay
≥98.0% (HPLC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
mp
186-188 °C
187-189 °C (lit.)
density
1.374 g/mL at 25 °C (lit.)
application(s)
cleaning products
cosmetics
environmental
food and beverages
personal care
format
neat
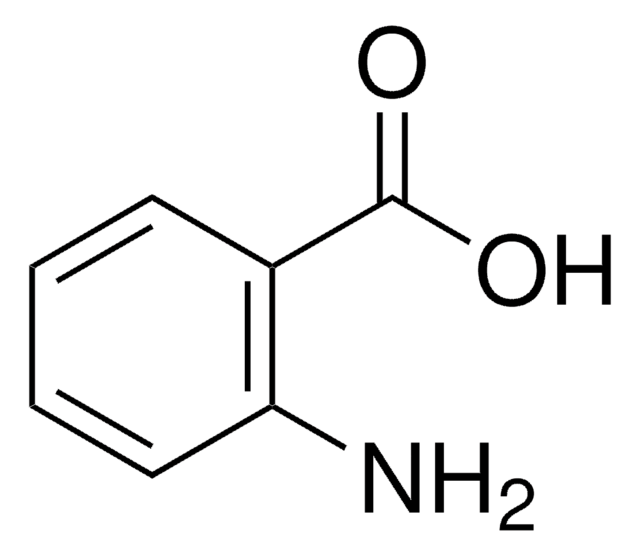
SMILES string
Nc1ccc(cc1)C(O)=O
InChI
1S/C7H7NO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4H,8H2,(H,9,10)
InChI key
ALYNCZNDIQEVRV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- 4-Aminobenzoic Acid (PABA) in Pharmaceutical Development: Recognized as a valuable biochemical research reagent, 4-aminobenzoic acid is extensively used in the synthesis of various pharmaceutical compounds. Its role as a building block in drug design is crucial for developing new therapeutic agents, particularly in the fields of dermatology and oncology (Clegg and Nichol, 2024).
- UV Protection Research: PABA is renowned for its UV-absorbing properties, making it a key ingredient in sunscreen formulations. Ongoing research evaluates its efficacy and safety, ensuring optimal protection against UV radiation and its potential role in preventing skin cancer (Wang et al., 2024).
- Enzyme Inhibitor Studies: As a nucleoside enzyme inhibitor, 4-aminobenzoic acid is pivotal in biochemical studies that explore the inhibition mechanisms of specific enzymes. These studies are fundamental for understanding disease pathways and developing enzyme-targeted therapies (Buch et al., 2024).
- Vitamin B10 Research: Also known as Vitamin B10, PABA is studied for its role in human nutrition and its therapeutic effects, including in the management of fibrotic skin disorders and hair growth promotion. Research continues to uncover the broader health benefits of this compound (Berto et al., 2024).
- High-Purity PABA for Laboratory Applications: In scientific research, high-purity 4-aminobenzoic acid is essential for conducting precise and controlled experiments, particularly in studies related to its pharmacological properties and its interaction with other biochemical substances in the body (Park et al., 2024).
Packaging
Other Notes
The collision cross section (CCS) measurement was provided by Waters Corporation, using the SYNAPT XS mass spectrometer.
For a description and overview of how ion mobility enables the measurement of the CCS of an ion visit ims.waters.com.
Further information on the SYNAPT XS mass spectrometer can be found on the IMS microsite and product webpage.
TWCCS measurements are expected to be within 2% of this reference value.
P/N 01973 is part of the Waters Extractables & Leachables UNIFI scientific library which can be downloaded from Waters Marketplace.
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
339.8 °F - closed cup
Flash Point(C)
171 °C - closed cup
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Separation of Uracil; p-Aminobenzoic acid; Acetylsalicylic acid; Dehydroacetic acid; Benzoic acid; Methyl paraben; 3-Fluorobenzoic acid
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service