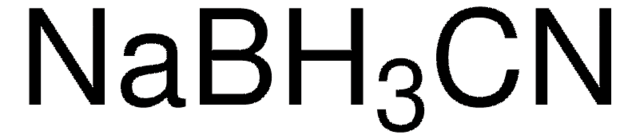8.06373
Sodium borohydride
fine granular for synthesis
Synonym(s):
Sodium borohydride, Sodium tetrahydridoborate
About This Item
Recommended Products
vapor pressure
<1 hPa ( 25 °C)
Quality Level
form
solid
autoignition temp.
220 °C
potency
160 mg/kg LD50, oral (Rat)
230 mg/kg LD50, skin (Rabbit)
expl. lim.
3.02 % (v/v)
reaction suitability
reagent type: reductant
pH
11 (20 °C, 10 g/L in H2O)
bp
500 °C (decomposition)
mp
>360 °C (slow decomposition)
transition temp
flash point 69 °C
density
1.07 g/cm3 at 20 °C
bulk density
350‑500 kg/m3
storage temp.
2-30°C
InChI
1S/BH4.Na/h1H4;/q-1;+1
InChI key
YOQDYZUWIQVZSF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Zero valent iron nanoparticles supported on mesoporous carbon (Starbon) by in situ reductions of Fe3+ ions.
- Ag nanoparticles on graphene using AgNO3 as a precursor.
- Ag-Pd/ZrO2 catalytic system applicable in plasmonic catalysis. It can also be used as a source to store as well as generate hydrogen for energy-carrier applications.
Analysis Note
Identity (ICP-OES): passes test
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Repr. 1B - Skin Corr. 1B - Water-react 1
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials, which set free flammable gases upon contact with water
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Fmoc resin cleavage and deprotection follows the difficult task of detaching the peptide from the resin support and removing all the side-chain protecting groups of the amino acid residues to yield the desired peptide.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service