567860
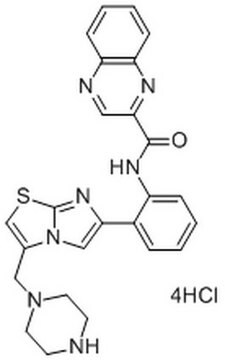
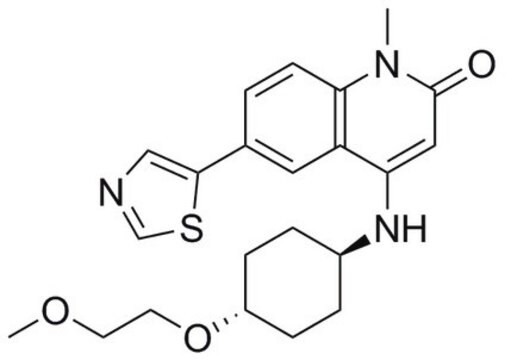
SRT1720
SRT1720, CAS 925434-55-5, is a cell-permeable inhibitor of the mitochondrial SIRT3. Inhibition is AceCS2-competitive (Ki = 0.56 µM; Km = 2.44 µM), but NAD+-uncompetitive (Ki = 0.34 µM; Km = 280 µM).
Synonym(s):
SRT1720, HAT Inhibitor XI, Histone Acetyltransferase Inhibitor XI, p300/CBP Inhibitor IX, N-(2-(3-(1-Piperazinylmethyl)imidazo[2,1-b]thiazol-6-yl)phenyl)-2-quinolinecarboxamide, Sirtuin-3 Inhibitor, SRT1720, SIRT3 Inhibitor II, HAT Inhibitor XI, Histone Acetyltransferase Inhibitor XI, p300/CBP Inhibitor IX, SIRT3 Inhibitor II, N-(2-(3-(1-Piperazinylmethyl)imidazo[2,1-b]thiazol-6-yl)phenyl)-2-quinolinecarboxamide, Sirtuin-3 Inhibitor, SRT1720
About This Item
Recommended Products
Quality Level
Assay
≥97% (HPLC)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
protect from light
color
yellow
solubility
DMSO: 2.5 mg/mL, clear, yellow
shipped in
ambient
storage temp.
−20°C
SMILES string
O=C(C1=NC(C=CC=C2)=C2N=C1)NC3=CC=CC=C3C4=CN5C(SC=C5CN6CCNCC6)=N4
InChI
1S/C25H23N7OS/c33-24(22-13-27-20-7-3-4-8-21(20)28-22)29-19-6-2-1-5-18(19)23-15-32-17(16-34-25(32)30-23)14-31-11-9-26-10-12-31/h1-8,13,15-16,26H,9-12,14H2,(H,29,33)
InChI key
IASPBORHOMBZMY-UHFFFAOYSA-N
General description
Packaging
Warning
Other Notes
Minor, R.K., et al. 2011. Sci. Rep.1, 70.
Huber, J.L., et al. 2010. Future Med. Chem.2, 1751
Pacholec, M., et al. 2010. J. Biol. Chem.285, 8340
Jin, L., et al. 2009. Protein Sci.18, 514
Feige, J.N., et al. 2008. Cell Metab.8, 347
Milne, J.C., et al. 2007. Nature450, 712.
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service