1.02282
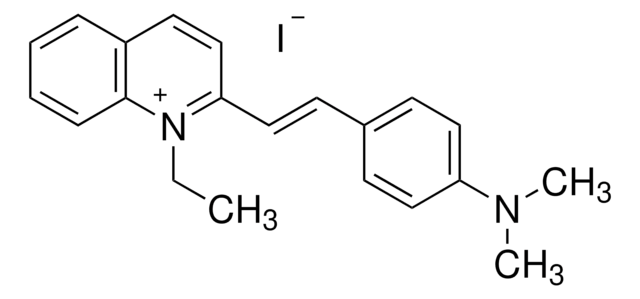
Quinaldine red
pH indicator, Reag. Ph Eur
Synonym(s):
Quinaldine red
About This Item
Recommended Products
Product Name
Quinaldine red, indicator Reag. Ph Eur
Agency
reag. Ph. Eur.
Quality Level
form
solid
impurities
≤5% Water (Karl Fischer)
visual transition interval
1.4-3.2, colorless to red
mp
240 °C (decomposition)
bulk density
290 kg/m3
λmax
528-533 nm in acetic acid
storage temp.
2-30°C
InChI
1S/C21H23N2.HI/c1-4-23-20(16-12-18-7-5-6-8-21(18)23)15-11-17-9-13-19(14-10-17)22(2)3;/h5-16H,4H2,1-3H3;1H/q+1;/p-1
InChI key
JOLANDVPGMEGLK-UHFFFAOYSA-M
Related Categories
Application
- Quinaldine Red as a fluorescent probe for determining the melting temperature (T(m)) of proteins: a simple, rapid and high-throughput assay.: This study presents Quinaldine Red as an effective fluorescent probe for determining the melting temperatures of proteins, offering a rapid and high-throughput method suitable for broad applications in biochemical research (Das et al., 2024).
- Red emitting fluorogenic dye as an efficient turn-on probe for milk allergen.: Explores the use of a red-emitting fluorogenic dye, likely involving Quinaldine Red, as a turn-on probe for detecting milk allergens, highlighting its potential in food safety and allergen detection (Chakraborty, 2022).
- A rapid, naked-eye detection of hypochlorite and bisulfite using a robust and highly-photostable indicator dye Quinaldine Red in aqueous medium.: Describes the application of Quinaldine Red for the visual detection of chemicals like hypochlorite and bisulfite, underlining its utility in environmental monitoring and chemical safety (Dutta et al., 2018).
- Self-Assembly and Formation of Chromonic Liquid Crystals from the Dyes Quinaldine Red Acetate and Pyronin Y.: Investigates the self-assembly properties of Quinaldine Red and its application in forming chromonic liquid crystals, demonstrating its potential in material science and nanotechnology (Magana et al., 2016).
Analysis Note
Transition range: pH 1.4 - pH 3.2 colourless - red
Absorption maximum λmax. (Acetic acid): 528 - 533 nm
Spec. Absorptivity A 1%/1cm (λmax; 0.005 g/l; acetic acid glacial; calculated on anhydrous substance): 1150 - 1480
Water (according to Karl Fischer): ≤ 5 %
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service