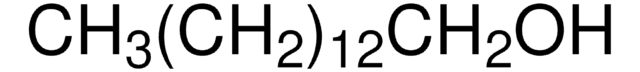All Photos(1)
About This Item
Linear Formula:
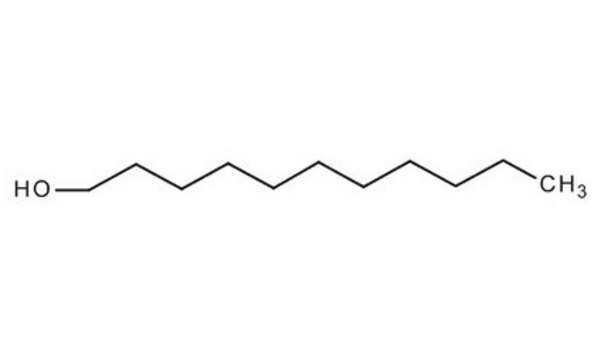
CH3(CH2)12OH
CAS Number:
Molecular Weight:
200.36
Beilstein:
1739991
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
6.9 (vs air)
Quality Level
Assay
97%
bp
155-156 °C/15 mmHg (lit.)
mp
29-34 °C (lit.)
density
0.822 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCCCCCCCO
InChI
1S/C13H28O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14/h14H,2-13H2,1H3
InChI key
XFRVVPUIAFSTFO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1-Tridecanol is a primary alcohol which can undergo oxidative conversion to:
It can also be used as a phase modifier for protonated trioctylamine in n-dodecane to study the speciation of vanadium (V) extracted from acidic sulfate media.
- 2,2,2-trifluoroethyl tridecanoate.
- Dodecanenitrile in the presence of molecular iodine in aq NH3.
It can also be used as a phase modifier for protonated trioctylamine in n-dodecane to study the speciation of vanadium (V) extracted from acidic sulfate media.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Speciation of vanadium (V) extracted from acidic sulfate media by trioctylamine in n-dodecane modified with 1-tridecanol.
Chagnes A, et al.
Hydrometallurgy, 104(1), 20-24 (2010)
Chemical degradation of trioctylamine and 1-tridecanol phase modifier in acidic sulfate media in the presence of vanadium (V).
Chagnes A, et al.
Hydrometallurgy, 105(3-4), 328-333 (2011)
Direct oxidative conversion of alcohols and amines to nitriles with molecular iodine and DIH in aq NH3.
Iida S and Togo H
Tetrahedron, 63(34), 8274-8281 (2007)
D A Haydon et al.
Biochimica et biophysica acta, 863(2), 337-340 (1986-12-16)
The effects of 0.09 saturated solutions of the n-alkanols n-hexanol to n-tridecanol on the surface (compensation) potential of lipid monolayers have been examined. Actions on monolayers spread from pure egg phosphatidylcholine have been compared with effects on a system containing
Facile oxidative conversion of alcohols to esters using molecular iodine.
Mori N and Togo H.
Tetrahedron, 61(24), 5915-5925 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service