P73803
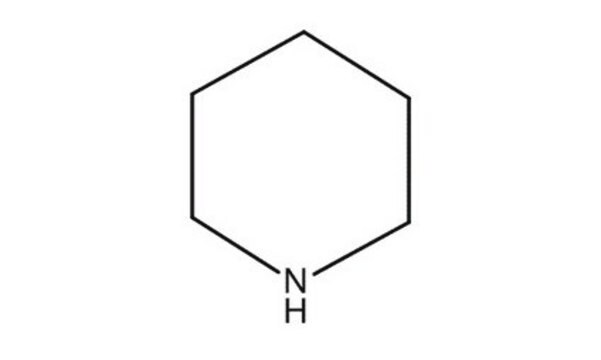
Pyrrolidine
99%
Synonym(s):
Tetrahydropyrrole, Tetramethyleneimine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C4H9N
CAS Number:
Molecular Weight:
71.12
Beilstein:
102395
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
2.45 (vs air)
Quality Level
vapor pressure
128 mmHg ( 39 °C)
49 mmHg ( 20 °C)
Assay
99%
autoignition temp.
653 °F
expl. lim.
10.6 %
refractive index
n20/D 1.443 (lit.)
density
0.852 g/mL at 25 °C (lit.)
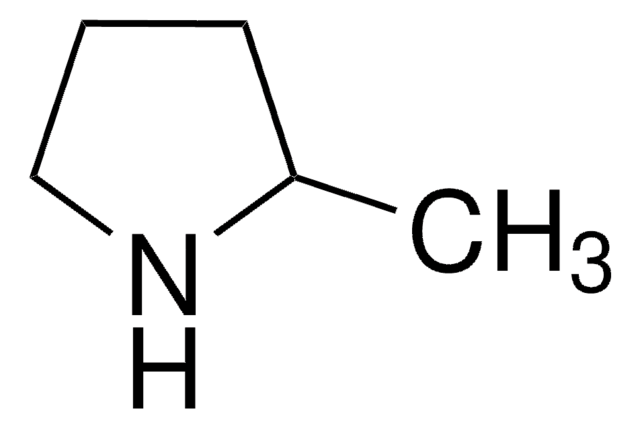
SMILES string
C1CCNC1
InChI
1S/C4H9N/c1-2-4-5-3-1/h5H,1-4H2
InChI key
RWRDLPDLKQPQOW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Pyrrolidine is a heterocyclic building block used in organic synthesis and a scaffold for biologically active compounds.
Application
Pyrrolidine has been used for the synthesis of N-benzoyl pyrrolidine from benzaldehyde via oxidative amination. It may be used as a catalyst for the synthesis of N-sulfinyl aldimines from carbonyl compounds and sulfonamides.
Pyrrolidine can also be used to synthesize:
Pyrrolidine can also be used to synthesize:
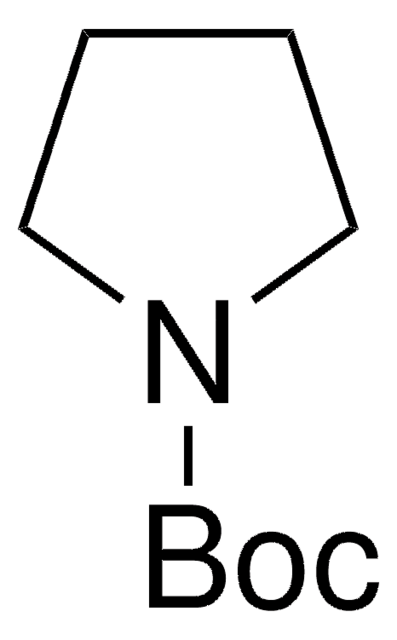
- Taddol-pyrrolidine phosphoramidite, a ligand for rhodium-catalyzed [2+2+2] cycloaddition of pentenyl isocyanate and 4- ethynylanisole.
- H,4 PyrrolidineQuin-BAM (′PBAM′), a selective catalyst for the aza-Henry addition of nitroalkanes to aryl aldimines.{88]
- 1,2,3,3a,4,9-Hexahydropyrrolo[2,1-b]quinazoline by reacting with o-aminobenzaldehyde.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1A
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
37.4 °F - closed cup
Flash Point(C)
3 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Preparation of N-Sulfinyl Aldimines using Pyrrolidine as Catalyst via Iminium Ion Activation
Morales S, et al.
Organic Syntheses, 94, 346-346 (2017)
Enantioselective Rhodium-Catalyzed [2+2+2] Cycloaddition of Pentenyl Isocyanate and 4-Ethynylanisole: Preparation and Use of Taddol-pyrrolidine Phosphoramidite
Oberg KM, et al.
Organic Syntheses, 91, 150-150 (2014)
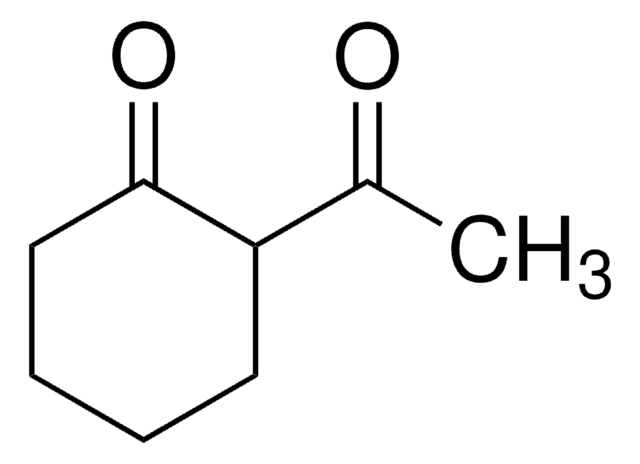
Metal?Free One?Pot Oxidative Amination of Aromatic Aldehydes: Conversion of Benzaldehyde to N?Benzoyl Pyrrolidine.
Ekoue?Kovi K & Wolf C
Organic Syntheses, 1-7 (2010)
o-Aminobenzaldehyde, Redox-Neutral Aminal Formation and Synthesis of Deoxyvasicinone
Zhang C, et al.
Organic Syntheses, 89, 274-274 (2012)
Pieter Van der Veken et al.
Journal of medicinal chemistry, 55(22), 9856-9867 (2012-11-06)
We have investigated the effect of regiospecifically introducing substituents in the P2 part of the typical dipeptide derived basic structure of PREP inhibitors. This hitherto unexplored modification type can be used to improve target affinity, selectivity, and physicochemical parameters in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)

![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)