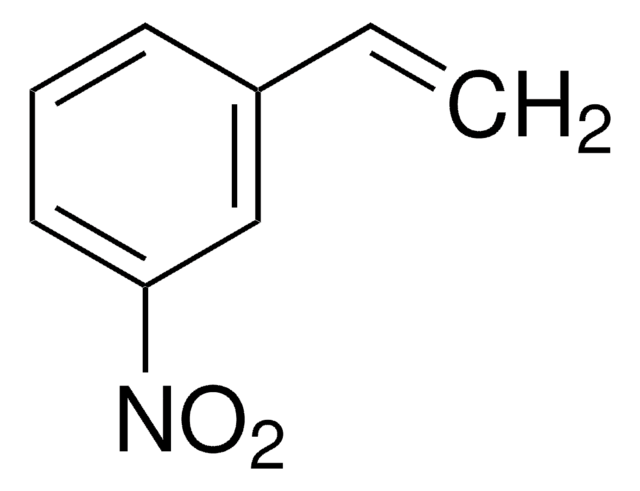
N26806
trans-β-Nitrostyrene
99%
Synonym(s):
trans-beta-Nitrostyrene, trans-1-Nitro-2-phenylethylene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH=CHNO2
CAS Number:
Molecular Weight:
149.15
Beilstein:
1210066
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
crystals
bp
250-260 °C (lit.)
mp
55-58 °C (lit.)
storage temp.
2-8°C
SMILES string
[O-][N+](=O)\C=C\c1ccccc1
InChI
1S/C8H7NO2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-7H/b7-6+
InChI key
PIAOLBVUVDXHHL-VOTSOKGWSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Wei-Ya Wang et al.
Biochemical pharmacology, 74(4), 601-611 (2007-07-03)
Protein tyrosine kinases have been known to be involved in regulation of platelet aggregation, suggesting a potential target for antiplatelet therapy. Our previous study showed that 3,4-methylenedioxy-beta-nitrostyrene (MNS) prevented platelet aggregation caused by various stimulators, and this action was accompanied
Catalytic asymmetric intermolecular Stetter reactions of enolizable aldehydes with nitrostyrenes: computational study provides insight into the success of the catalyst.
Daniel A DiRocco et al.
Angewandte Chemie (International ed. in English), 51(10), 2391-2394 (2012-01-28)
Kamal Nain Singh et al.
Bioorganic & medicinal chemistry letters, 22(13), 4225-4228 (2012-06-08)
An efficient asymmetric Michael addition of cyclic ketones to β-nitrostyrenes using secondary diamine as an organocatalyst derived from l-proline and (R)-α-methylbenzyl amine has been described. This pyrrolidine based catalyst 1 was found to be very effective to synthesize various γ-nitrocarbonyl
Yan Huang et al.
The Journal of organic chemistry, 74(3), 1252-1258 (2008-12-31)
A convenient and efficient way for the highly diastereoselective synthesis of beta-substituted-alpha,gamma-diaminobutyric acids and pyrrolidines containing multichiral centers has been well-developed. Michael addition of chiral tricyclic iminolactones 1 and 2 to nitroalkenes afforded the adducts in good yields (up to
Jens Martin Werner et al.
Journal of pharmacological and toxicological methods, 57(2), 131-137 (2007-12-19)
Induction of apoptosis is perceived as the main intention of drug regimens for tumour therapy. Thus, the concentration- and time-dependence of drug-induced apoptosis should be carefully evaluated for experimental as well as for standard anti-tumour agents. A main feature of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service