All Photos(1)
About This Item
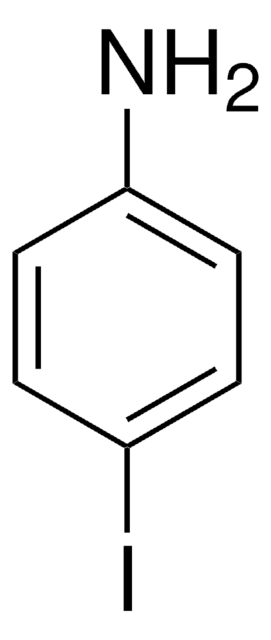
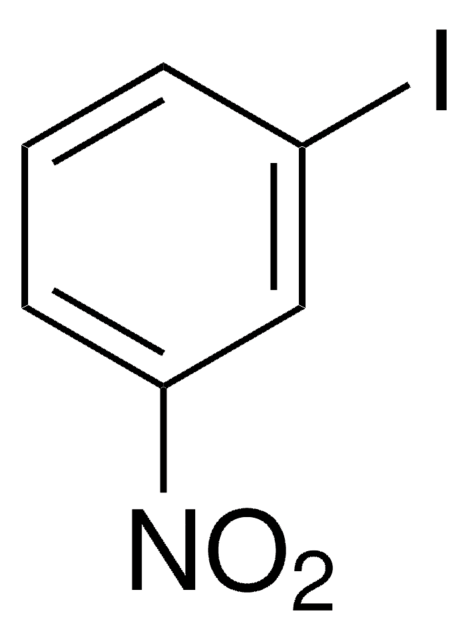
Linear Formula:
IC6H4NH2
CAS Number:
Molecular Weight:
219.02
Beilstein:
636058
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.682 (lit.)
bp
145-146 °C/15 mmHg (lit.)
mp
21-24 °C (lit.)
density
1.821 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
Nc1cccc(I)c1
InChI
1S/C6H6IN/c7-5-2-1-3-6(8)4-5/h1-4H,8H2
InChI key
FFCSRWGYGMRBGD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Karsten Franke et al.
Environmental science & technology, 42(11), 4083-4087 (2008-07-02)
A new method is described for radiolabeling humic substances (HS) with iodine radioisotopes. The method radiolabels the electron-rich aromatic moieties of HS with the 3-[125I]iodobenzenediazonium ion via azocoupling. The method uses four steps: (i) 3-aminobenzenetrimethylstannane is synthesized and isolated by
Karin Dooleweerdt et al.
Organic letters, 11(1), 221-224 (2008-11-28)
A Pd-catalyzed, one-pot, two-step synthesis of 2-amidoindoles from ynamides and o-iodoanilines is reported. A key highlight of this sequence is that after the Sonogashira reaction, intramolecular cyclization to the indole occurs spontaneously without activation of the alkyne.
Toshio Nishikawa et al.
Bioscience, biotechnology, and biochemistry, 66(10), 2273-2278 (2002-11-27)
An alpha-C-iodoethynylglucose derivative was coupled with an L-serine-derived zinc-copper reagent to give alpha-C-glucosylpropargyl glycine, which underwent palladium catalyzed-heteroannulation with o-iodoaniline to give not alpha-C-glucosyl-tryptophan but alpha-C-glucosyl-iso-tryptophan. This is the first observation of complete reverse regioselectivity to Larock's proposal.
Fangguo Ye et al.
The Journal of organic chemistry, 72(9), 3218-3222 (2007-04-06)
The palladium-catalyzed cyclocarbonylation reaction of o-iodoanilines with allenes and CO in 1-butyl-3-methylimidazolium hexafluorophosphate afforded 3-methylene-2,3-dihydro-1H-quinolin-4-ones in moderate to excellent yields under a low pressure (5 atm) of CO. The ionic liquid, as the solvent and promoter, enhances the efficiency of
L A Khawli et al.
International journal of radiation applications and instrumentation. Part B, Nuclear medicine and biology, 19(3), 297-301 (1992-04-01)
Biotinyl-m-[125I]iodoanilide (BIA) was synthesized by coupling biotin to m-[125I]iodoaniline via a mixed anhydride reaction. m-[125I]Iodoaniline was produced from the tin precursor, which was prepared using a palladium catalyzed reaction of hexabutylditin with m-bromoaniline. The radioiodinated BIA derivative is characterized by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service