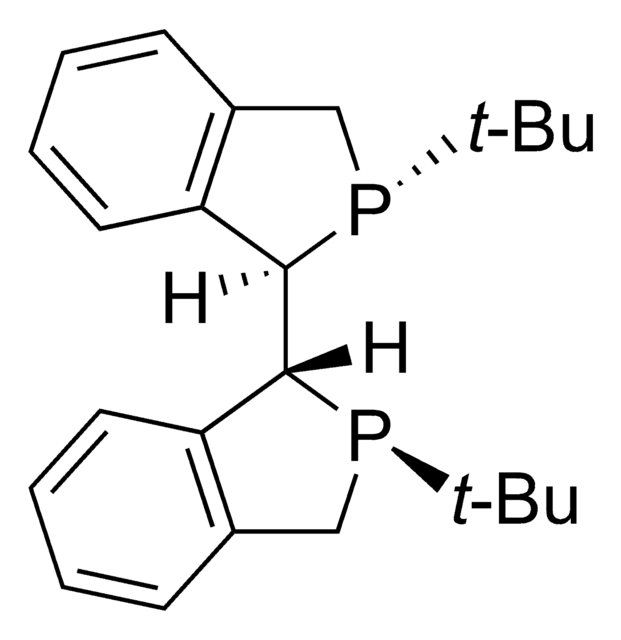
912468
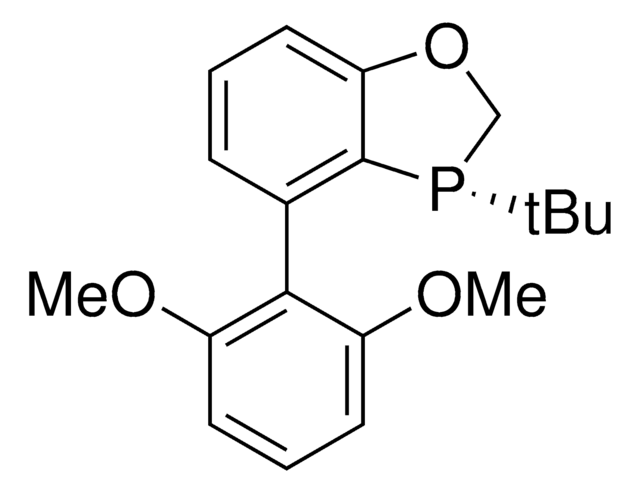
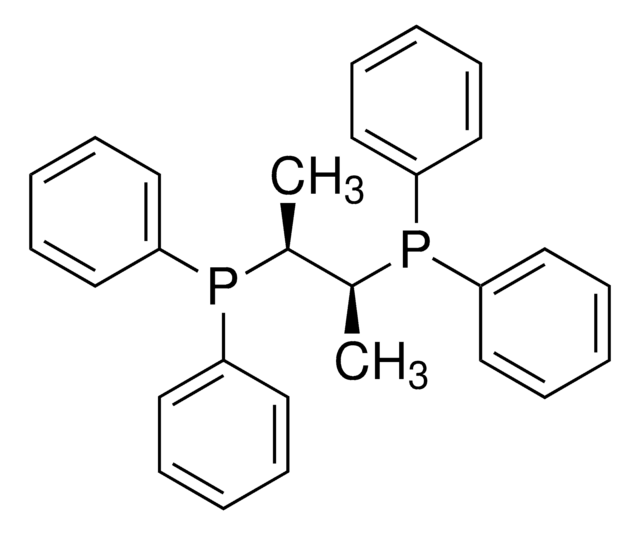
(2S,3S)-iPr-BIDIME
≥95%
Synonym(s):
(2S,3S)-3-(tert-butyl)-4-(2,6-dimethoxyphenyl)-2-isopropyl-2,3-dihydrobenzo[d][1,3]oxaphosphole
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C22H29O3P
CAS Number:
Molecular Weight:
372.44
UNSPSC Code:
12352200
Recommended Products
Assay
≥95%
form
powder
optical purity
ee: ≥99% (HPLC)
reaction suitability
reagent type: ligand
functional group
phosphine
Application
(2S,3S)-iPr-BIDIME is a P-chiral monophosphorus ligand used for the transition metal-catalyzed asymmetric Suzuki-Miyaura and hydroboration reactions.
Legal Information
Sold in collaboration with Zejun Pharmaceuticals
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Guangqing Xu et al.
Journal of the American Chemical Society, 136(2), 570-573 (2013-10-24)
Efficient asymmetric Suzuki-Miyaura coupling reactions are employed for the first time in total syntheses of chiral biaryl natural products korupensamine A and B in combination with an effective diastereoselective hydrogenation, allowing ultimately a concise and stereoselective synthesis of michellamine B.
Naifu Hu et al.
Journal of the American Chemical Society, 137(21), 6746-6749 (2015-05-06)
The rhodium-catalyzed asymmetric hydroboration of α-arylenamides with BI-DIME as the chiral ligand and (Bpin)2 as the reagent yields for the first time a series of α-amino tertiary boronic esters in good yields and excellent enantioselectivities (up to 99% ee).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service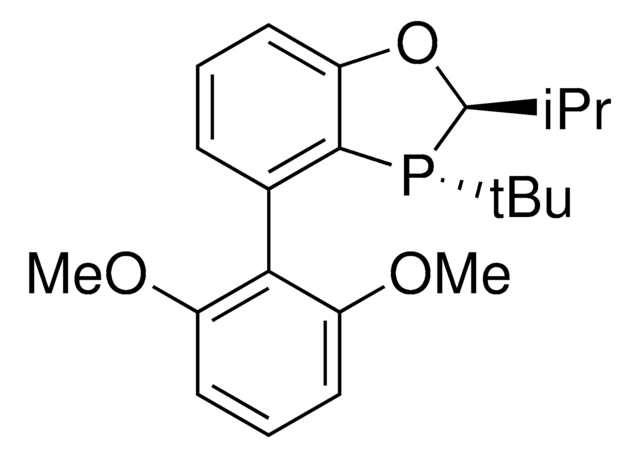
![(+)-1,2-Bis[(2S,5S)-2,5-dimethylphospholano]benzene kanata purity](/deepweb/assets/sigmaaldrich/product/structures/319/912/cec7b70f-bf7c-4a96-9f11-a73ae892e34c/640/cec7b70f-bf7c-4a96-9f11-a73ae892e34c.png)