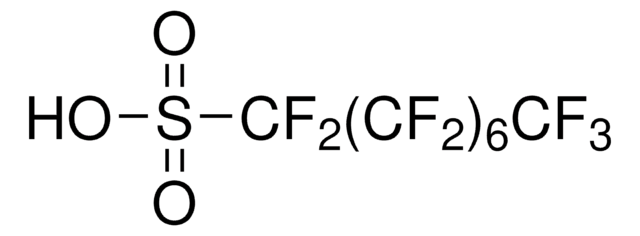86909
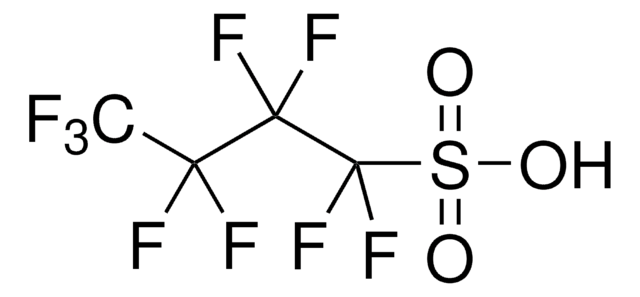
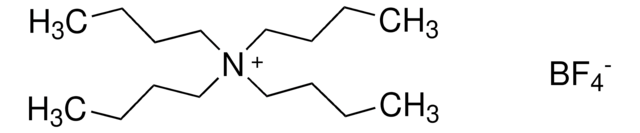
Tetrabutylammonium nonafluorobutanesulfonate
≥98.0%
Synonym(s):
Perfluorobutanesulfonic acid tetrabutylammonium salt, Tetrabutylammonium perfluorobutanesulfonate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3CH2CH2CH2)4N(CF3CF2CF2CF2SO3)
CAS Number:
Molecular Weight:
541.56
Beilstein:
5219877
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.0%
form
powder
mp
50-53 °C
SMILES string
CCCC[N+](CCCC)(CCCC)CCCC.[O-]S(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F
InChI
1S/C16H36N.C4HF9O3S/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;5-1(6,3(9,10)11)2(7,8)4(12,13)17(14,15)16/h5-16H2,1-4H3;(H,14,15,16)/q+1;/p-1
InChI key
VECGWISURDHBJL-UHFFFAOYSA-M
General description
Tetrabutylammonium nonafluorobutanesulfonate is a fluorinated ionic liquid (FIL). Its solid-fluid transition and nanoscale structuring have been studied using differential scanning calorimetry (DSC), rheology techniques and molecular dynamic simulations.
Other Notes
Ionic liquid
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fluorination effects on the thermodynamic, thermophysical and surface properties of ionic liquids.
Vieira NSM, et al.
The Journal of Chemical Thermodynamics, 97, 354-361 (2016)
Superhydrophobic properties of electrodeposited fluorinated polypyrenes
Chagas, Gabriela Ramos, et al.
Journal of Fluorine Chemistry, 193, 73-81 (2017)
On the Formation of a Third, Nanostructured Domain in Ionic Liquids.
Pereiro AB, et al.
The Journal of Physical Chemistry B, 117(37), 10826-10833 (2013)
C.F. Poole et al. et al.
Molten Salt Techniques, 4, 41-41 (1989)
Which structural features stand behind micelization of ionic liquids? Quantitative Structure-Property Relationship studies
Barycki, Maciej, Anita Sosnowska, and Tomasz Puzyn
Journal of Colloid and Interface Science, 487, 475-483 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service