79330
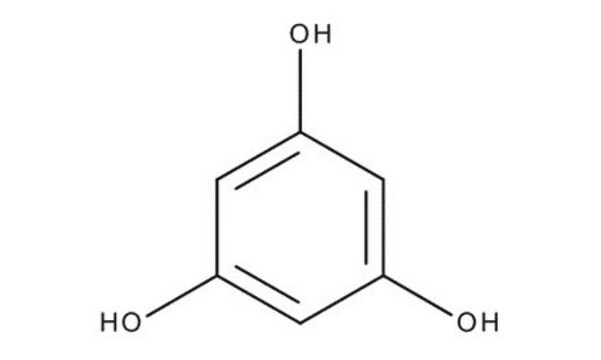
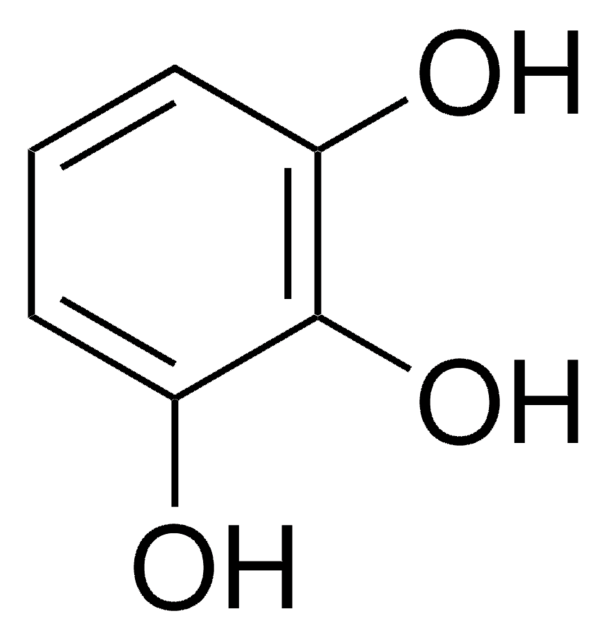
Phloroglucinol
≥99.0% (HPLC)
Synonym(s):
1,3,5-Trihydroxybenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H6O3
CAS Number:
Molecular Weight:
126.11
Beilstein:
1341907
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99.0% (HPLC)
form
solid
impurities
diresorcin, none detected
≤2% water
mp
215-220 °C
SMILES string
Oc1cc(O)cc(O)c1
InChI
1S/C6H6O3/c7-4-1-5(8)3-6(9)2-4/h1-3,7-9H
InChI key
QCDYQQDYXPDABM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Phloroglucinol (phlo) is a phenol derivative that shows cyctoprotective effect from oxidative damage by enhancing the activity of cellular catalase.
- It can react with benzaldehyde derivatives to form phloroglucinol-based microporous polymeric organic frameworks (phlo-POF) with potential applications in ion-exchange and gas adsorption.
- Phlo can also be used to prepare synthetic analogs of A-type proanthocyanidins (PACs) such as 2,8-dioxabicyclo[3.3.1]nonane derivatives by reacting with the corresponding flavylium salts.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cytoprotective effect of phloroglucinol on oxidative stress induced cell damage via catalase activation
Kang KA, et al.
Journal of Cellular Biochemistry, 97(3), 609-620 (2006)
Efficient and Rapid Photocatalytic Reduction of Hexavalent Chromium Achieved by a Phloroglucinol-Derived Microporous Polymeric Organic Framework Solid
Kostas V, et al.
The Journal of Physical Chemistry C, 121(13), 7303-7311 (2017)
Thermodynamic Stability of Flavylium Salts as a Valuable Tool To Design the Synthesis of A-Type Proanthocyanidin Analogues
Alejo-Armijo A, et al.
The Journal of Organic Chemistry, 83(19), 12297-12304 (2018)
Claudia Birkemeyer et al.
Metabolites, 10(9) (2020-09-17)
Accumulation of biologically active metabolites is a specific feature of plant biochemistry, directing the use of plants in numerous applications in the pharmaceutical and food industries. Among these substances, the plethora of phenolic compounds has attracted particular interest among researchers.
Anne M Vissers et al.
Phytochemical analysis : PCA, 28(6), 487-495 (2017-06-15)
Phlorotannins are complex mixtures of phloroglucinol oligomers connected via C-C (fucols) or C-O-C (phlorethols) linkages. Their uniformity in subunits and large molecular weight hamper their structural analysis. Despite its commercial relevance for alginate extraction, phlorotannins in Laminaria digitata have not
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service