754242
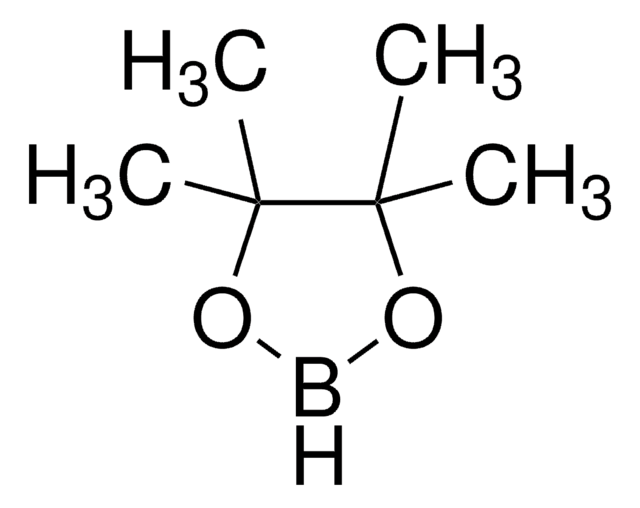
Tetrahydroxydiboron
95%
Synonym(s):
BBA, Bis-Boric acid, Diboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
Empirical Formula (Hill Notation):
B2H4O4
CAS Number:
Molecular Weight:
89.65
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
mp
>385 °C
SMILES string
OB(O)B(O)O
InChI
1S/B2H4O4/c3-1(4)2(5)6/h3-6H
InChI key
SKOWZLGOFVSKLB-UHFFFAOYSA-N
Related Categories
Application
Tetrahydroxydiboron (BBA) is an efficient borylating agent that can be used to borylate a wide variety of aryl and heteroaryl substrates with low Pd- and Ni-catalyst loads.
Other reactions employing BBA as a reagent:
Other reactions employing BBA as a reagent:
- Palladium-catalyzed boronation of vinyl cyclopropane, vinyl aziridine, and allyl acetate substrates.
- To facilitate catalytic transfer hydrogenations of simple alkenes and alkynes.
- Selective reducing agent for in situ N-oxide reduction of pyridine-N-oxides.
- As a substitute to bis(pinacolato) diboron for Miyaura borylation.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Scope of the Palladium-Catalyzed Aryl Borylation Utilizing Bis-Boronic Acid.
Molander GA, et al.
Journal of the American Chemical Society, 134, 11667-11673 (2012)
Scope of the Two-Step, One-Pot Palladium-Catalyzed Borylation/ Suzuki Cross-Coupling Reaction Utilizing Bis-Boronic Acid.
Molander GA, et al.
The Journal of Organic Chemistry, 77, 8678-8688 (2012)
Development and Scale-up of an Efficient Miyaura Borylation Process Using Tetrahydroxydiboron.
Gurung S R, et al.
Organic Process Research & Development, 21(1), 65-74 (2016)
Tetrahydroxydiboron-Mediated Palladium-Catalyzed Transfer Hydrogenation and Deuteriation of Alkenes and Alkynes Using Water as the Stoichiometric H or D Atom Donor.
Cummings S P, et al.
Journal of the American Chemical Society, 138(19), 6107-6110 (2016)
Rapid and Selective in situ Reduction of Pyridine-N-oxides with Tetrahydroxydiboron.
Londregan A T
Synlett, 24(20), 2695-2700 (2013)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 754242-1G | |
| 754242-1KG | 4061826735862 |
| 754242-100G | 4061833552186 |
| 754242-250MG | 4061833339862 |
| 754242-25G | 4061833552193 |
| 754242-5G | 4061832907918 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)