694037
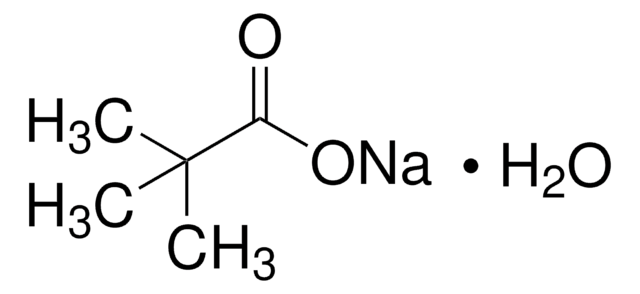
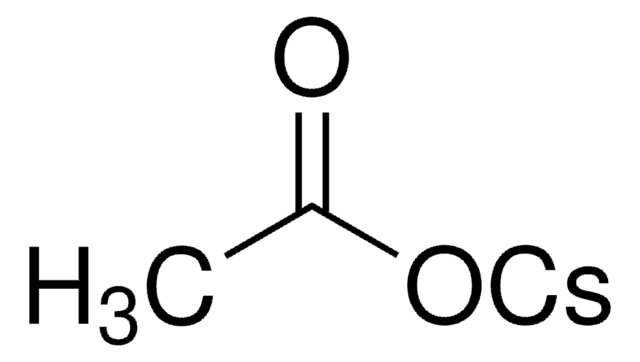
Cesium pivalate
98%
Synonym(s):
Cesium 2,2-dimethylpropanoate, Cesium trimethylacetate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C5H9O2Cs
CAS Number:
Molecular Weight:
234.03
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
mp
344-348 °C
SMILES string
[Cs+].CC(C)(C)C([O-])=O
InChI
1S/C5H10O2.Cs/c1-5(2,3)4(6)7;/h1-3H3,(H,6,7);/q;+1/p-1
InChI key
LGVUAXNPXVXCCW-UHFFFAOYSA-M
General description
Cesium pivalate is an organic base widely used in palladium-catalyzed cross-coupling and carbonylation reactions due to its solubility in organic solvents.
Application
Cesium pivalate can be used as a base to synthesize:
- Fluoren-9-one derivatives by cyclocarbonylation of o-halobiaryls in the presence of palladium catalyst.
- Fused heterocycles (dihydrobenzofurans and indolines) from o-bromo phenol and aniline precursors via Pd-catalyzed intramolecular coupling of two C(sp3)-H bonds.
- Amides and esters derivatives containing a quaternary β-carbon atom by Pd-catalyzed C-H activation and amino/alkoxycarbonylation reaction.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M A Campo et al.
Organic letters, 2(23), 3675-3677 (2000-11-14)
The synthesis of various substituted fluoren-9-ones has been accomplished by a novel palladium-catalyzed cyclocarbonylation of o-halobiaryls. The cyclocarbonylation of 4'-substituted-2-iodobiphenyls produces very high yields of 2-substituted fluoren-9-ones bearing either electron-donating or electron-withdrawing substituents. 3'-Substituted 2-iodobiphenyls afford in excellent yields with
Tomáš Čarný et al.
Angewandte Chemie (International ed. in English), 59(43), 18980-18984 (2020-07-22)
The 1,4-palladium shift strategy allows the functionalization of remote C-H bonds that are difficult to reach directly. Reported here is a domino reaction proceeding by C(sp3 )-H activation, 1,4-palladium shift, and amino- or alkoxycarbonylation, which generates a variety of amides
Zubaoyi Yi et al.
The Journal of organic chemistry, 82(13), 6946-6957 (2017-06-16)
Pd-catalyzed arylation or benzylation of nitroazoles using aryl sulfonates or benzyl acetates is described. Electronically varied aryl tosylates and mesylates, as well as benzyl acetates, afford the arylated and benzylated products. Arylation of nitrobenzene is also reported. The relative rate
Aditya L Gottumukkala et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(11), 3091-3095 (2011-02-10)
Ace of base: A catalytic system is presented that, solely by choice of the base, selectively switches between conjugate addition and the Mizoroki-Heck reaction of aryl halides with Michael acceptors (see scheme; R, R' = alkyl, aryl). For conjugate addition
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)