652571
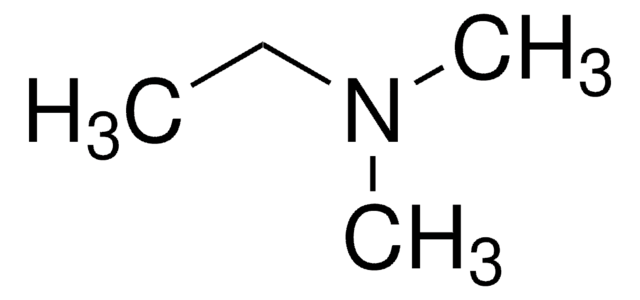
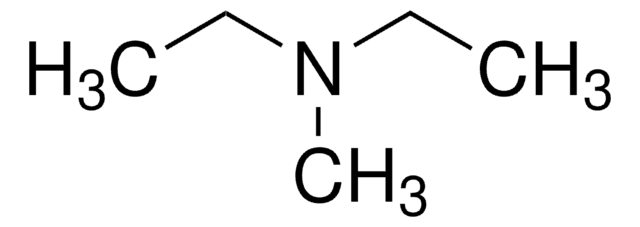
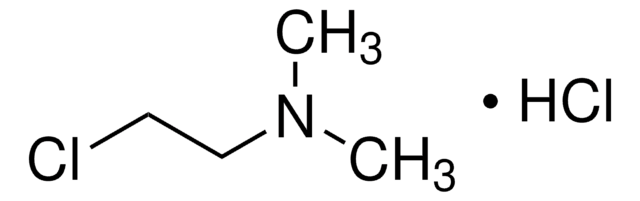
N,N-Dimethylethylamine
≥99%
Synonym(s):
N-Ethyldimethylamine, DMEA
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)2NC2H5
CAS Number:
Molecular Weight:
73.14
Beilstein:
1696893
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
8.09 psi ( 20 °C)
Quality Level
Assay
≥99%
refractive index
n20/D 1.372 (lit.)
bp
36-38 °C (lit.)
mp
−140 °C (lit.)
density
0.675 g/mL at 25 °C (lit.)
SMILES string
CCN(C)C
InChI
1S/C4H11N/c1-4-5(2)3/h4H2,1-3H3
InChI key
DAZXVJBJRMWXJP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
N,N-Dimethylethylamine (DMEA) is generally used to prepare water-soluble quaternary ammonium salts. It facilitates lithium hexamethyldisilazide (LiHMDS) mediated enolization of highly substituted aryl ketones. Additionally, DMEA is also used as an organic solvent in synthetic chemistry.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
-18.4 °F - closed cup
Flash Point(C)
-28 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Lithium Hexamethyldisilazide-Mediated Enolization of Highly Substituted Aryl Ketones: Structural and Mechanistic Basis of the E/Z Selectivities.
Mack KA, et al.
Journal of the American Chemical Society, 139(35), 12182-12189 (2017)
A theophylline based copper N-heterocyclic carbene complex: synthesis and activity studies in green media.
Szadkowska A, et al.
Royal Society of Chemistry Advances, 6(50), 44248-44253 (2016)
Sodium Diisopropylamide in Tetrahydrofuran: Selectivities, Rates, and Mechanisms of Alkene Isomerizations and Diene Metalations.
Algera RF, et al.
Journal of the American Chemical Society, 139(33), 11544-11549 (2017)
Sodium Diisopropylamide in N, N-Dimethylethylamine: Reactivity, Selectivity, and Synthetic Utility.
Ma Y, et al.
The Journal of Organic Chemistry, 81(22), 11312-11315 (2016)
Highly Stereoselective Synthesis of Tetrasubstituted Acyclic All-Carbon Olefins via Enol Tosylation and Suzuki-Miyaura Coupling.
Li BX, et al.
Journal of the American Chemical Society, 139(31), 10777-10783 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service