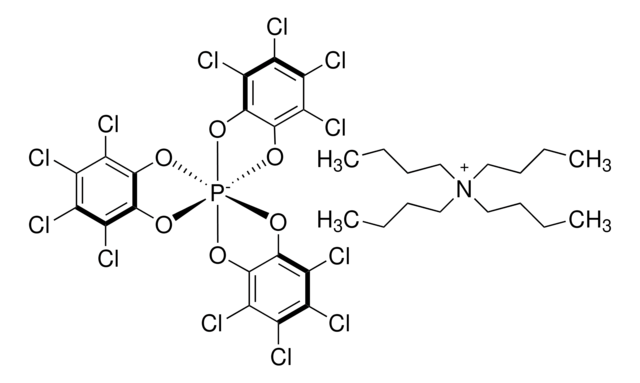
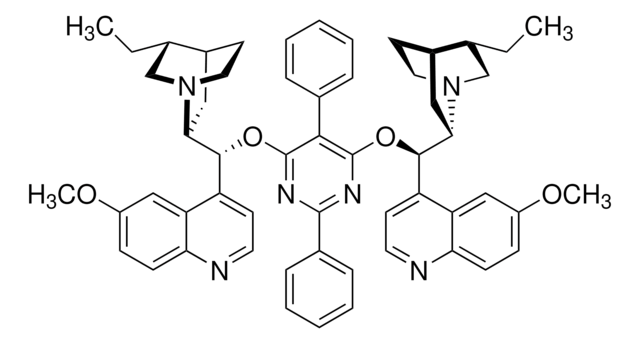
About This Item
Recommended Products
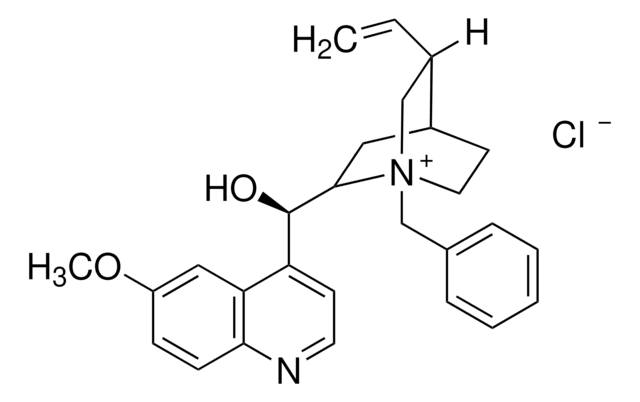
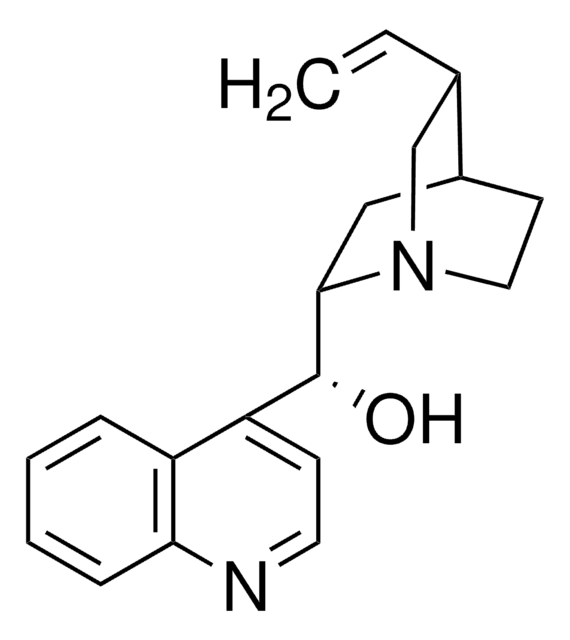
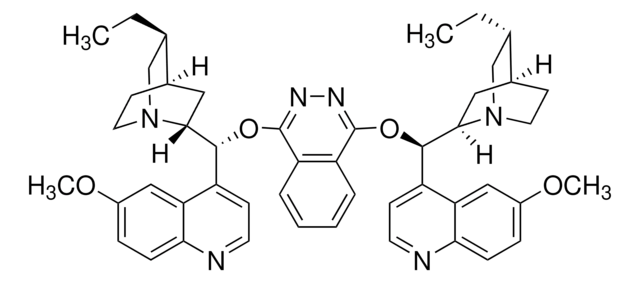
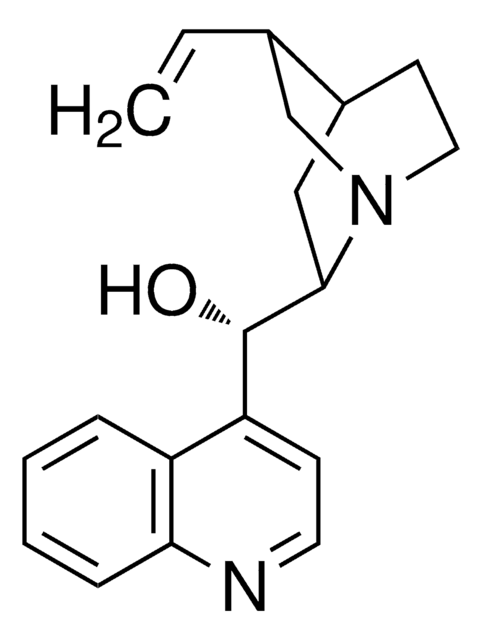
Assay
97%
optical activity
[α]20/D −138°, c = 1 in chloroform
mp
190 °C (dec.) (lit.)
Related Categories
General description
Application
- To prepare various 4-(bromomethyl)benzenesulfonamides, which are employed as catalysts for asymmetric benzylation reactions.
- As a phase transfer catalyst in the synthesis of organogelators via Michael addition reaction.
- To catalyze the enantioselective alkylation of tert-butyl glycinate-benzophenone Schiff base furnishing the corresponding chiral α-amino acid.
- To resolve symmetric biaryldiols via formation of inclusion complex with diols.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Asymmetric phase transfer catalysis using the Maruoka catalysts has proven to be an ideal method for the enantioselective preparation of natural and unnatural α-alkyl and α,α-dialkyl-α-amino acids from glycine derivati
Asymmetric phase-transfer catalysis (PTC) has been recognized as a “green” alternative to many homogeneous synthetic organic transformations, and has found widespread application. Synthetically modified cinchona alkaloids are typical chiral organocatalysts used in asymmetric PTC.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(11bS)-(+)-4,4-Dibutyl-4,5-dihydro-2,6-bis(3,4,5-trifluorophenyl)-3H-dinaphth[2,1-c:1′,2′-e]azepinium bromide](/deepweb/assets/sigmaaldrich/product/structures/230/279/5c1a7e7e-f791-4612-9920-56c6d7c4f735/640/5c1a7e7e-f791-4612-9920-56c6d7c4f735.png)
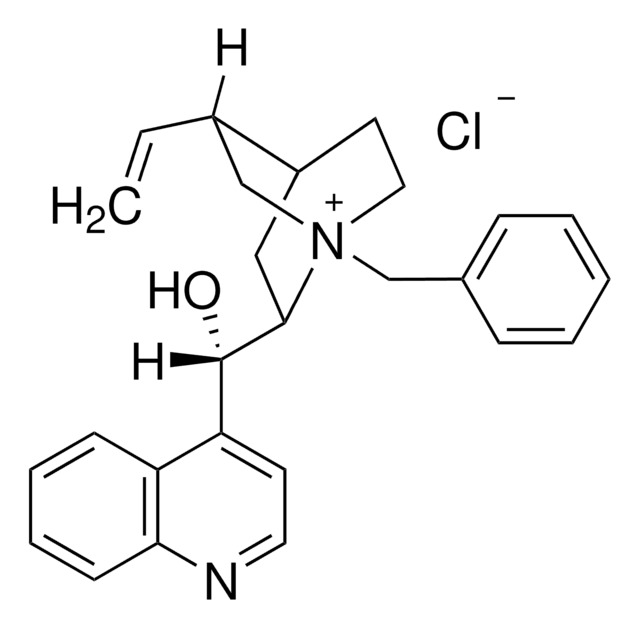
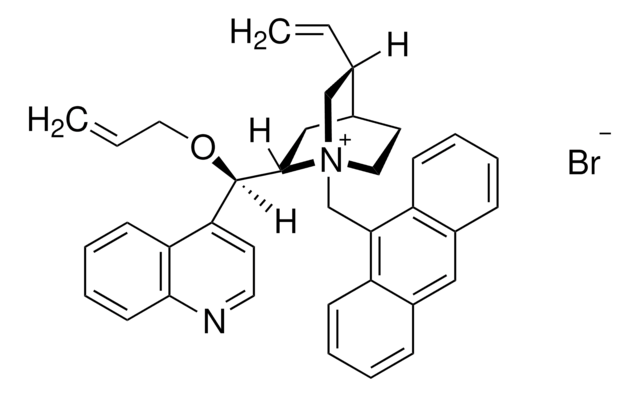
![(11bR)-(–)-4,4-Dibutyl-4,5-dihydro-2,6-bis(3,4,5-trifluorophenyl)-3H-dinaphth[2,1-c:1′,2′-e]azepinium bromide Nagase purity](/deepweb/assets/sigmaaldrich/product/structures/147/005/c2a47bf2-5270-4469-9f3e-138f76f8f4c3/640/c2a47bf2-5270-4469-9f3e-138f76f8f4c3.png)