469920
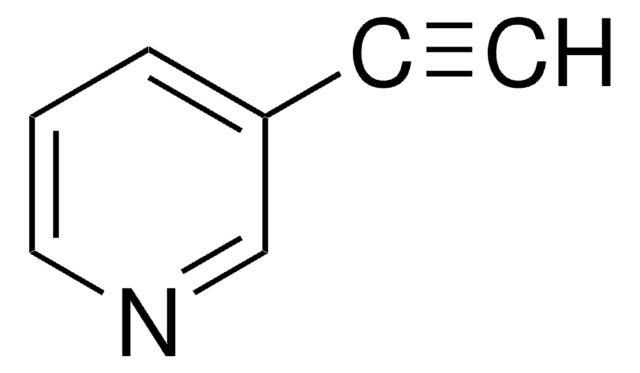
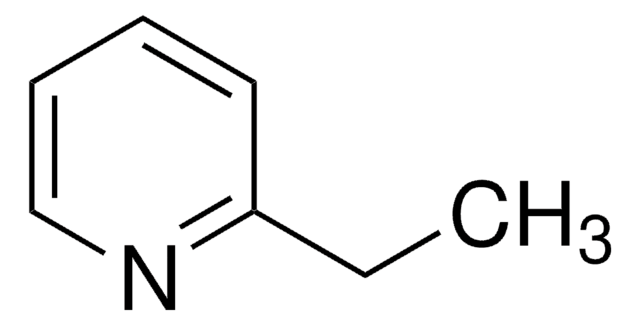
2-Ethynylpyridine
98%
Synonym(s):
2-Pyridinylethyne, 2-Pyridylacetylene, Pyridin-2-ylacetylene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H5N
CAS Number:
Molecular Weight:
103.12
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.56 (lit.)
bp
85 °C/12 mmHg (lit.)
density
1.021 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C#Cc1ccccn1
InChI
1S/C7H5N/c1-2-7-5-3-4-6-8-7/h1,3-6H
InChI key
NHUBNHMFXQNNMV-UHFFFAOYSA-N
General description
2-Ethynylpyridine is a pyridine derivative.
Application
2-Ethynylpyridine has been used to synthesize:
- poly(2-ethynyl-N-iodopyridinium iodide), via in-situ uncatalyzed polymerization
- poly[2-ethynyl-N-(2-furoyl)pyridinium chloride
- poly[2-ethynyl-N-(propylsulfonate)pyridinium betaine]
- 4-(2′-pyridyl)-1,2,3-triphospholide anion, via reaction with anionic heptaphosphide clusters
- poly[N-(carboxymethyl)-2-ethynylpyridinium bromide], a new water-soluble ionic conjugated polymer
- self-doped ionic conjugated polymer, poly(2-ethynylpyridinimum N-benzoylsulfonate).
Other Notes
It may darken in storage with no loss in purity.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yeong-Soon Gal et al.
Journal of nanoscience and nanotechnology, 12(5), 4361-4364 (2012-08-03)
A new water-soluble ionic conjugated polymer, poly[N-(carboxymethyl)-2-ethynylpyridinium bromide], was prepared by the activated polymerization of 2-ethynylpyridine by using bromoacetic acid. This polymerization proceeded well in mild reaction conditions without any additional initiator or catalyst. The polymer structure was characterized by
Yeong-Soon Gal et al.
Journal of nanoscience and nanotechnology, 14(10), 8028-8032 (2015-05-07)
Poly(2-ethynyl-N-iodopyridinium iodide) [PEIPI] was easily prepared via in-situ uncatalyzed polymerization of 2-ethynylpyridine by using iodine. The activated acetylenic bond of 2-ethynyl-N-iodopyridinium iodide formed at the initial reaction time was assumed to be susceptible to linear polymerization, followed by an identical
Robert S P Turbervill et al.
Inorganic chemistry, 52(9), 5527-5534 (2013-04-24)
Reactions between anionic heptaphosphide clusters ([P7](3-)/[HP7](2-)) and 2-ethynylpyridine yielded the 4-(2'-pyridyl)-1,2,3-triphospholide anion ([P3C2H(2-C5H4N)](-); 1). This species was isolated as a compositionally pure [K(2,2,2-crypt)](+) salt in moderate yields. Preliminary coordination studies of 1 toward Mo(CO)6 or Mo(py)3(CO)3 (py = pyridine) afforded
Yeong-Soon Gal et al.
Journal of nanoscience and nanotechnology, 14(8), 6247-6250 (2015-05-06)
A new ionic polyacetylene derivative with furoyl substituents was prepared by the uncatalyzed polymerization of 2-ethynylpyridine by using 2-furoyl chloride in high yield. The polymer structure was characterized by such instrumental methods as NMR, IR, and UV-visible spectroscopies to have
Cecilia Zavala-Tecuapetla et al.
Pharmacological reports : PR, 66(5), 927-930 (2014-08-26)
Action of an antagonist of metabotropic glutamate receptors subtype 5 MTEP was studied in a model of complex partial seizures. Dorsal hippocampus of rat pups 12, 18 and 25 days old was stimulated six times with 10-min intervals. MTEP (20
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service