467030
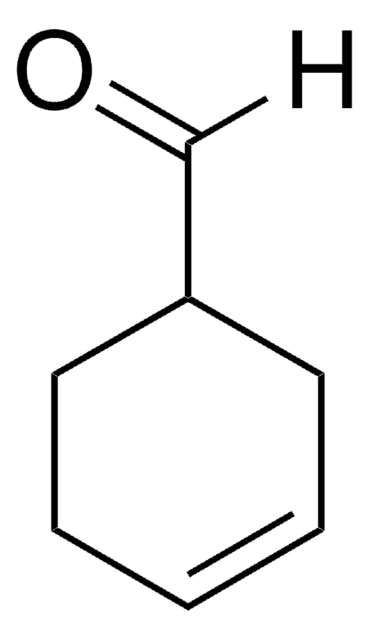
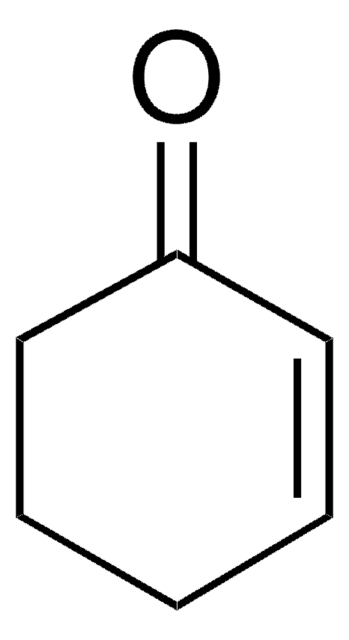
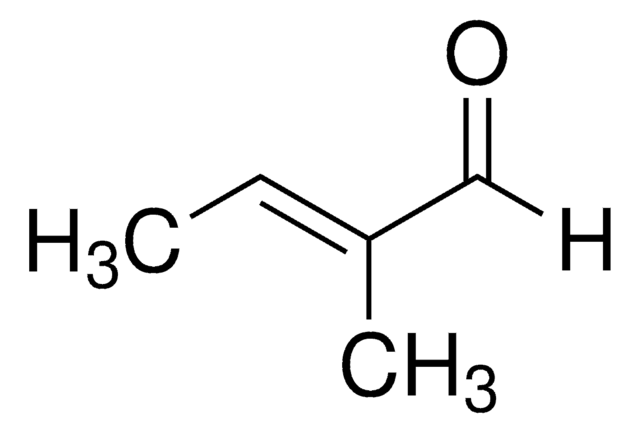
1-Cyclohexene-1-carboxaldehyde
97%
Synonym(s):
Δ1-Tetrahydrobenzaldehyde, 1-Cyclohexenecarboxaldehyde, 1-Cyclohexenylaldehyde, 1-Formyl-1-cyclohexene, 1-Formylcyclohexene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H9CHO
CAS Number:
Molecular Weight:
110.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
bp
61 °C/10 mmHg (lit.)
density
0.966 g/mL at 25 °C (lit.)
functional group
aldehyde
SMILES string
O=CC1=CCCCC1
InChI
1S/C7H10O/c8-6-7-4-2-1-3-5-7/h4,6H,1-3,5H2
InChI key
OANSOJSBHVENEI-UHFFFAOYSA-N
General description
1-Cyclohexene-1-carboxaldehyde is an α,β-unsaturated aldehyde. It participates in the synthesis of benzopyrans.
Application
1-Cyclohexene-1-carboxaldehyde may be used in the synthesis of azomethine imines.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
149.0 °F - closed cup
Flash Point(C)
65 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Feng Shi et al.
Tetrahedron letters, 50(28), 4067-4070 (2010-02-18)
A [3+2] 1,3-dipolar cycloaddition reaction of arynes with stable azomethine imines has been developed. The reaction rapidly assembles tricyclic pyrazoloindazolone derivatives in moderate yields under mild reaction conditions.
Environmentally benign, one-pot synthesis of pyrans by domino Knoevenagel/6p-electrocyclization in water and application to natural products.
Jung EJ, et al.
Green Chemistry, 12(11), 2003-2011 (2010)
Efficient and general method for the synthesis of benzopyrans by ethylenediamine diacetate-catalyzed reactions of resorcinols with α, β-unsaturated aldehydes. One step synthesis of biologically active (?)-confluentin and (?)-daurichromenic acid.
Lee YR, et al.
Tetrahedron Letters, 46(44), 7539-7543 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service