All Photos(1)
About This Item
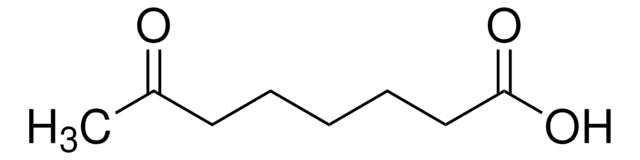
Linear Formula:
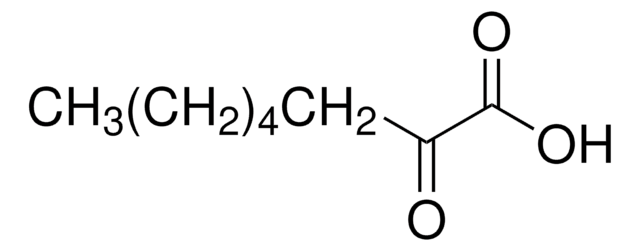
CH3CO(CH2)4CO2H
CAS Number:
Molecular Weight:
144.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
90%
form
solid
bp
158-162 °C/9 mmHg (lit.)
mp
35-37 °C (lit.)
density
1.059 g/mL at 25 °C (lit.)
SMILES string
CC(=O)CCCCC(O)=O
InChI
1S/C7H12O3/c1-6(8)4-2-3-5-7(9)10/h2-5H2,1H3,(H,9,10)
InChI key
IZOQMUVIDMLRDC-UHFFFAOYSA-N
Related Categories
General description
6-Oxoheptanoic acid is a monocarboxylic acid with acyl functional group. Mass spectrometric characterization of 6-oxoheptanoic acid by electrospray ionization coupled to a triple quadrupole and TOF analyzer hybrid system has been reported.
Application
6-Oxoheptanoic acid may be used in the following studies:
- As ketone linker used for the conjugation of hydrazide derivatives to proteins.
- Synthesis of N-(2-propynyl)-6-oxoheptanamide.
- Synthesis of adenosine triphosphate derivative.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible, corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mass spectrometric characterization of small oxocarboxylic acids and gas phase ion fragmentation mechanisms studied by electrospray triple quadrupole-MS/MS-TOF system and DFT theory.
Kanawati B, et al.
International Journal of Mass Spectrometry, 266(1), 97-113 (2007)
A Safavy et al.
Bioconjugate chemistry, 10(1), 18-23 (1999-01-20)
A procedure utilizing an activated ester approach for conjugation of unprotected hydroxamic acids to antibodies and peptides was recently reported. Here, an alternative method with advantages over the activated ester strategy is described. This protocol utilizes the hydrazone formation between
Synthesis of methylketone containing nucleoside triphosphates for RNA labelling
Trevisiol E, et al.
Tetrahedron, 56(35), 6501-6510 (2000)
Erik Selander et al.
Proceedings of the National Academy of Sciences of the United States of America, 112(20), 6395-6400 (2015-04-29)
Interactions among microscopic planktonic organisms underpin the functioning of open ocean ecosystems. With few exceptions, these organisms lack advanced eyes and thus rely largely on chemical sensing to perceive their surroundings. However, few of the signaling molecules involved in interactions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service