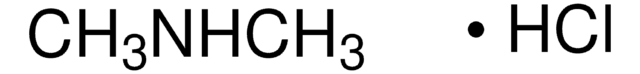399019
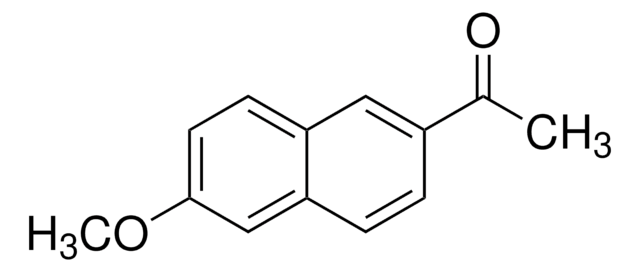
6′-Methoxy-2′-acetonaphthone
98%
Synonym(s):
1-(6-Methoxy-2-naphthalenyl)ethanone, 2-Acetyl-6-methoxynaphthalene, NSC 105564
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3OC10H6COCH3
CAS Number:
Molecular Weight:
200.23
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
107-109 °C (lit.)
functional group
ketone
SMILES string
COc1ccc2cc(ccc2c1)C(C)=O
InChI
1S/C13H12O2/c1-9(14)10-3-4-12-8-13(15-2)6-5-11(12)7-10/h3-8H,1-2H3
InChI key
GGWCZBGAIGGTDA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
6′-Methoxy-2′-acetonaphthone (6-methoxy-2-naphthylacetic acid ) is metabolite of nabumetone, a phototoxic nonsteroidal antiinflammatory drug.1
6′-Methoxy-2′-acetonaphthone is suitable for use in the synthesis of 6-methoxy-2-naphthylacetic acid. It may be used in the preparation of fluorogenic aldol sensors.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
F Boscá et al.
Photochemistry and photobiology, 71(2), 173-177 (2000-02-25)
Nabumetone is a phototoxic nonsteroidal antiinflammatory drug used for the treatment of osteoarthritis. However, nabumetone is considered a prodrug with its metabolite 6-methoxy-2-naphthylacetic acid the active form. Photophysical and photochemical studies on this metabolite have been undertaken. It undergoes photodecarboxylation
M Nobilis et al.
Journal of pharmaceutical and biomedical analysis, 32(4-5), 641-656 (2003-08-06)
The disposition of the non-steroidal anti-inflammatory drug (NSAID) nabumetone after a single oral dose administration of nabumetone tablets to humans and minipigs was investigated. Nabumetone is a prodrug, which is metabolized in the organism to the principal pharmacodynamically active metabolite
B List et al.
Proceedings of the National Academy of Sciences of the United States of America, 95(26), 15351-15355 (1998-12-23)
The synthesis of novel fluorogenic retro-aldol substrates for aldolase antibody 38C2 is described. These substrates are efficiently and specifically processed by antibody aldolases but not by natural cellular enzymes. Together, the fluorogenic substrates and antibody aldolases provide reporter gene systems
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service