392189
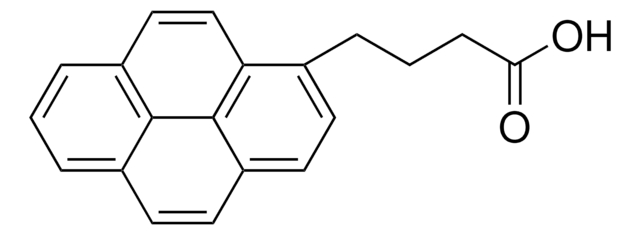
1-Pyreneacetic acid
97%
Synonym(s):
(1-Pyrenyl)acetic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H12O2
CAS Number:
Molecular Weight:
260.29
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
210-212 °C (dec.) (lit.)
SMILES string
OC(=O)Cc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C18H12O2/c19-16(20)10-14-7-6-13-5-4-11-2-1-3-12-8-9-15(14)18(13)17(11)12/h1-9H,10H2,(H,19,20)
InChI key
SDJCLYBBPUHKCD-UHFFFAOYSA-N
General description
1-Pyreneacetic acid is a negatively charged pyrene derivative. It has been proposed as titrating reagent for the standardization titration of Grignard reagents and n-butyl lithium (n-BuLi).
Application
1-Pyreneacetic acid is suitable for use in the following studies:
- Synthesis of N-(2-(methylthio)ethyl)-2-(pyren-1-yl)acetamide, a pyrene amide based Pd2+ probe.
- Synthesis of pyrene-modified β-cyclodextrin.
- To functionalize single walled carbon nanotube field effect transistors (CNT FETs).
- As an agent for characterizing grafting degrees and reactivity of the ester functionalized polypropylenes.
- Synthesis sawhorse-type diruthenium tetracarbonyl complexes.
- Synthesis of (±)-2-(1-pyrenyl)propionic acid, a chiral carboxylic acid.
- Reversible noncovalent functionalization of single walled carbon nanotubes (SWNTs).
- Preparation of peptide nucleic acid (PNA) probes.
- As an internal reference compound in the assessment of solid phase reaction by HPLC-UV.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jan Spengler et al.
ACS combinatorial science, 15(5), 229-234 (2013-03-26)
Here we evaluated the use of internal reference compounds for the rapid assessment of reactions performed in solid-phase. An internal reference compound (commercially available) was bound to the resin, together with the substrate, and cleaved with the products after completion
Sawhorse-type diruthenium tetracarbonyl complexes derived from pyrenyl-carboxylic acids.
Johnpeter JP and Therrien B.
Inorgorganica Chimica Acta, 405, 437-443 (2013)
Gabriela Ramos Chagas et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 18(23), 3429-3436 (2017-09-01)
A smart stimuli-responsive surface was fabricated by the electro-copolymerization of pyrene monomers followed by base and acid treatment. Copolymers of pyrenes bearing fluorinated chains (Py-nF
Alex Manicardi et al.
Beilstein journal of organic chemistry, 10, 1495-1503 (2014-08-28)
Pyrene derivatives can be incorporated into nucleic acid analogs in order to obtain switchable probes or supramolecular architectures. In this paper, peptide nucleic acids (PNAs) containing 1 to 3 1-pyreneacetic acid units (PNA1-6) with a sequence with prevalence of pyrimidine
Murphy PJ.
Organophosphorus Reagents: A Practical Approach in Chemistry, 8-8 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service