375721
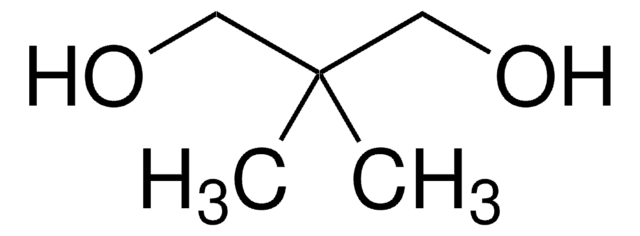
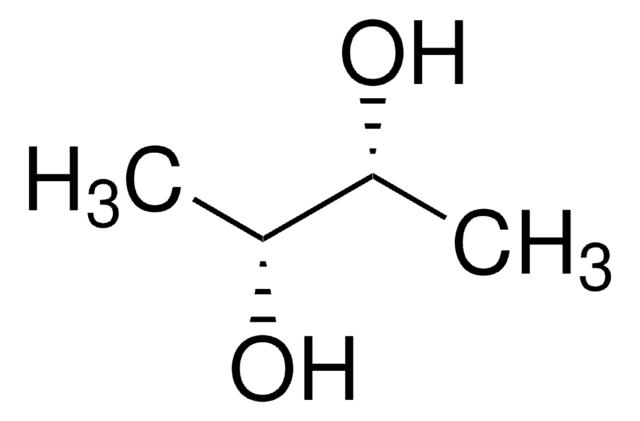
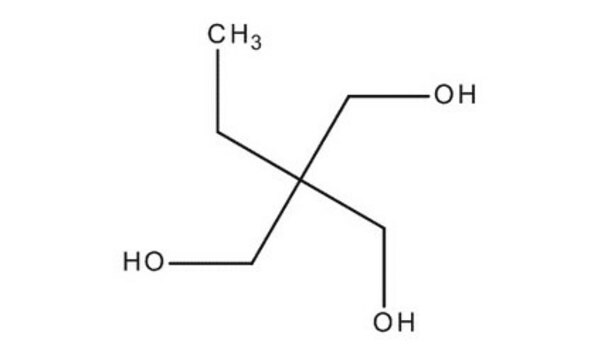
2-Methyl-1,3-propanediol
99%
Synonym(s):
1,3-Dihydroxy-2-methylpropane, 2-Methylpropan-1,3-diol, Methylpropanediol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH(CH2OH)2
CAS Number:
Molecular Weight:
90.12
Beilstein:
1731422
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
vapor density
3.2 (vs air)
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.445 (lit.)
bp
123-125 °C/20 mmHg (lit.)
mp
−91 °C (lit.)
density
1.015 g/mL at 25 °C (lit.)
SMILES string
CC(CO)CO
InChI
1S/C4H10O2/c1-4(2-5)3-6/h4-6H,2-3H2,1H3
InChI key
QWGRWMMWNDWRQN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Methyl-1,3-propanediol is a biodegradable, colorless glycol. This low molecular weight compound has no significant hazard potential and is widely used in polymer and coating applications.
Application
2-Methyl-1,3-propanediol is used:
- In the preparation of solid polymer electrolytes for lithium metal batteries.
- To obtain methyl methacrylate (precursor for polymethyl methacrylate glass) via a partial oxidation process.
- In the synthesis of biodegradable thermoplastic elastomer by condensation reaction.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
260.6 °F - closed cup
Flash Point(C)
127 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J Fowles et al.
Regulatory toxicology and pharmacology : RTP, 91, 240-248 (2017-11-04)
2-methyl 1,3-propandiol (MPD) is a low molecular weight, colorless glycol used in polymer and coating applications. The log Kow of -0.6 suggests partitioning to aqueous phases with a low concern for possible bioaccumulation. MPD was found to be inherently biodegradable.
Zhi-Yu Yang et al.
Soft matter, 16(2), 402-410 (2019-12-04)
A series of bio-based poly(propylene-co-2-methyl-1,3-propanediol 2,5-furandicarboxylate) (PPMF) copolyesters, with various compositions from poly(propylene 2,5-furandicarboxylate) (PPF) to poly(2-methyl-1,3-propylene 2,5-furandicarboxylate) (PMePF), were synthesized by conventional melt polymerization. The effects of the substituent group to PPF on the thermal properties, mechanical properties, and
Juri Maeda et al.
Biochimica et biophysica acta, 1861(8), 2112-2118 (2017-04-30)
Due to the strict enantioselectivity of firefly luciferase, only d-luciferin can be used as a substrate for bioluminescence reactions. Unfortunately, luciferin racemizes easily and accumulation of nonluminous l-luciferin has negative influences on the light emitting reaction. Thus, maintaining the enantiopurity
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 375721-3L | 4061831835045 |
| 375721-1L | 4061831835038 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service