367095
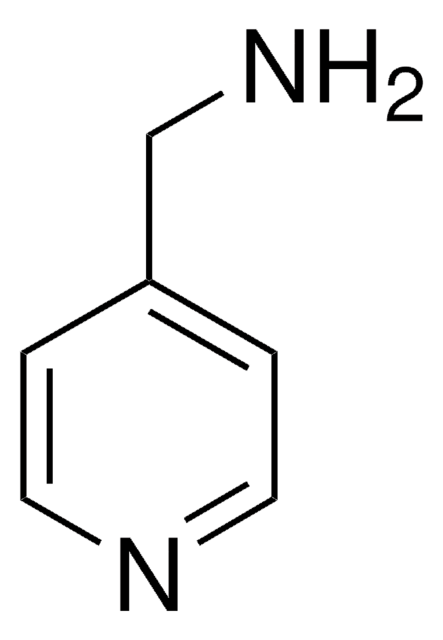
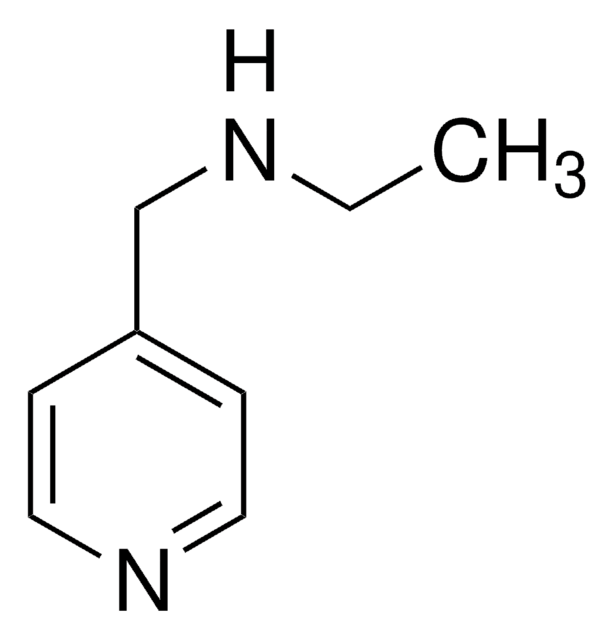
4-(Ethylaminomethyl)pyridine
97%
Synonym(s):
N-(Pyridin-4-ylmethyl)ethanamine, N-Ethyl-4-pyridinemethanamine, NSC 85903
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H12N2
CAS Number:
Molecular Weight:
136.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.516 (lit.)
density
0.979 g/mL at 25 °C (lit.)
SMILES string
CCNCc1ccncc1
InChI
1S/C8H12N2/c1-2-9-7-8-3-5-10-6-4-8/h3-6,9H,2,7H2,1H3
InChI key
ZBAMQLFFVBPAOX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-(Ethylaminomethyl)pyridine is a secondary amine.
Application
4-(Ethylaminomethyl)pyridine is suitable for use in the synthesis of sodium dithiocarbamate ligands. It may be used in the synthesis of 4-(ethylaminodithiocarbamate) methylpyridine diorganotin derivatives. It may be used in the synthesis of {[ethyl(pyridin-4-ylmethyl)carbamothioyl]sulfanido-κ2 S,S′}(1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O)potassium.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
208.4 °F - closed cup
Flash Point(C)
98 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and structural characterization of diorganotin dithiocarbamates from 4-(ethylaminomethyl) pyridine.
Barba V, et al.
Heteroatom Chem., 23(5), 422-428 (2012)
Adduct effects on structure and luminescence in a series of new dithiocarbamatogold (I) complexes. T
Han S, et al.
Transition Met. Chem. (London), 36(7), 691-697 (2011)
Hadi D Arman et al.
Acta crystallographica. Section E, Structure reports online, 69(Pt 9), m479-m480 (2014-01-16)
The asymmetric unit of title salt co-crystal, [K(C9H11N2S2)(C12H24O6)], comprises a K(+) cation, an (-)S2CN(Et)py anion and a 18-crown-6 mol-ecule. Substantial delocalization of π-electron density is evident in the di-thio-carbamate anion, as indicated by the equivalent C-S bond lengths. The K(+)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service