All Photos(1)
About This Item
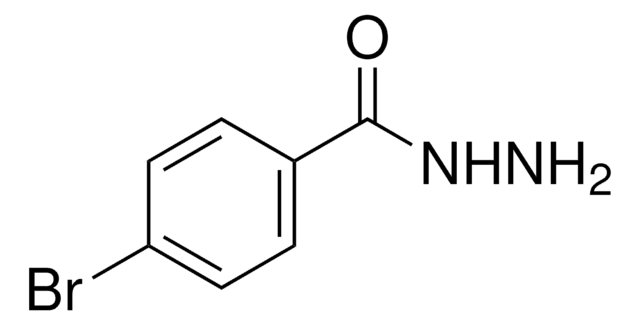
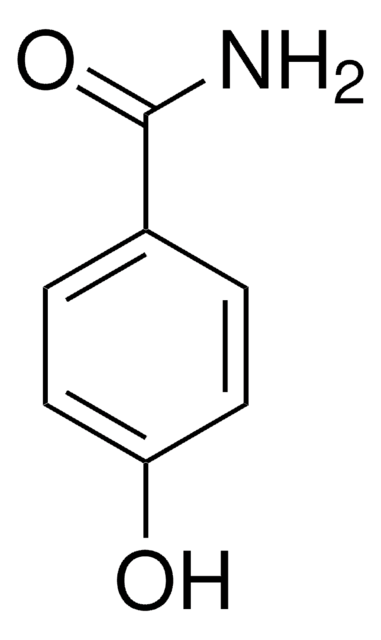
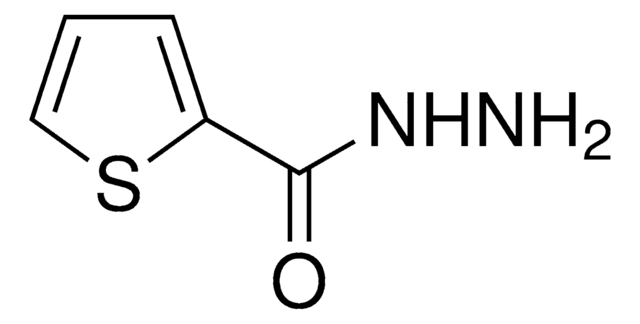
Linear Formula:
ClC6H4CONHNH2
CAS Number:
Molecular Weight:
170.60
Beilstein:
511154
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
162-165 °C (lit.)
functional group
amine
chloro
hydrazine
SMILES string
NNC(=O)c1ccc(Cl)cc1
InChI
1S/C7H7ClN2O/c8-6-3-1-5(2-4-6)7(11)10-9/h1-4H,9H2,(H,10,11)
InChI key
PKBGHORNUFQAAW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Effect of acidic catalyst on the reaction of 4-chlorobenzhydrazide and β-naphthol in water has been investigated.
Application
4-Chlorobenzhydrazide has been used in preparation of:
- rod-shaped mesogenic hydrazide derivatives via Schotten-Baumann reaction with 4-n-alkoxybenzoyl chloride
- 4-methoxybenzaldehyde-4-chlorophenyl-1-carbonyl hydrazone
- 4-hydroxybenzaldehyde-4-chlorophenyl-1-carbonylhydrazone
- 2-nitrobenzaldehyde-4-chlorophenyl-1-carbonylhydrazone
- benzaldehyde-4-chlorophenyl-1-carbonylhydrazone
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Minoo Dabiri et al.
Bioorganic & medicinal chemistry letters, 18(1), 436-438 (2007-12-07)
Alkyl- or aryl-14H-dibenzo[a,j]xanthene derivatives are synthesized efficiently by the reaction of beta-naphthol and aliphatic and aromatic aldehydes in the presence of KAl(SO4)2 x 12 H2O (alum) under aqueous condition at 100 degrees C. Different types of aromatic and aliphatic aldehydes
Bent-shaped mesogenic oxadiazole and thiadiazole derivatives from rod-shaped mesogenic hydrazide containing polar chloro group.
Prajapati AK and Modi V.
Liq. Cryst., 37(4), 407-415 (2010)
Synthesis of benzaldehyde substituted phenyl carbonyl hydrazones and their formylation using Vilsmeier-Haack reaction.
Rajput AP and Rajput AS.
International Journal of PharmTech Research, 1(4), 1605-1611 (2009)
Jagvir Singh et al.
Bioinorganic chemistry and applications, 2012, 104549-104549 (2012-11-06)
N-substituted pyridine hydrazide (pyridine-2-carbonyl chloride and 4-chloro-benzoic acid hydrazide) undergoes hydrazide formation of the iminic carbon nitrogen double bond through its reaction with cobalt(II), nickel(II), and copper(II) metal salts in ethanol which are reported and characterized based on elemental analyses
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service