223220
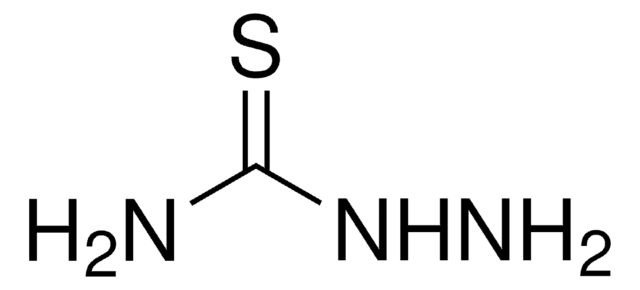
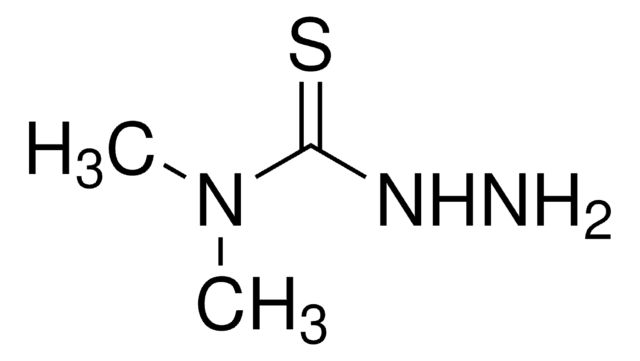
Thiocarbohydrazide
98%
Synonym(s):
Thiocarbonyldihydrazide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(NH2NH)2CS
CAS Number:
Molecular Weight:
106.15
Beilstein:
506657
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39093513
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
171-174 °C (dec.) (lit.)
functional group
amine
hydrazine
thiourea
SMILES string
NNC(=S)NN
InChI
1S/CH6N4S/c2-4-1(6)5-3/h2-3H2,(H2,4,5,6)
InChI key
LJTFFORYSFGNCT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Review of transition metal complexes with thiocarbohydrazides: This comprehensive review discusses the coordination chemistry of thiocarbohydrazides with various metals, highlighting their relevance in the synthesis of complex metal compounds used in catalysis and pharmaceutical research (Aly et al., 2023).
- Pd-doped nanocomposites for organometallic catalysis: Thiocarbohydrazide was employed in the fabrication of Pd-doped SBA-15 nanocomposites, applied as catalysts in the synthesis of organometallic compounds, showcasing its utility as a catalyst support material (Kalhor and Dadras, 2021).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M A Badawy et al.
Archiv der Pharmazie, 324(6), 349-351 (1991-06-01)
Heterocycles containing the indole ring system include some novel pharmacologically active compounds. Isatin and its N-acetylisatin are extremely versatile intermediates in the construction of a variety of heterocyclic systems when reacted with thiosemicarbazide derivatives. Literature survey revealed various interesting reactions
E A Dunnebier et al.
Hearing research, 90(1-2), 139-148 (1995-10-01)
The stereociliar structures of the guinea-pig cochlear organ of Corti were studied at low-voltage (1-5 kV) with field-emission scanning electron microscope (SEM) using various pre- and post-fixation methods, such as OTOTO (OsO4/thiocrbohydrazide/OsO4/thiocarbohydrazide/OsO4) and TAO (tannic acid/arginine/OsO4), and different dissection procedures
D P Singh et al.
Journal of enzyme inhibition and medicinal chemistry, 22(2), 177-182 (2007-05-24)
A novel series of complexes of the type [M(TML)X2]; where TML is Tetradentate Macrocyclic Ligand; M = Co(II), Ni(II), Cu(II), Zn(II)or Cd(II); X = Cl-, CH3COO- or NO3- have been synthesized by template condensation of glyoxal and thiocarbohydrazide in the
D Levanon et al.
The Histochemical journal, 31(1), 71-73 (1999-07-16)
Samples from seven sectors of the rabbit knee articular cartilage were shaved and prepared for the scanning electron microscope using either tannic acid, thiocarbohydrazide or nothing (control). Surface morphology was found to be more typical to a given sector and
Kamaleddin Haj Mohammad Ebrahim Tehrani et al.
Iranian journal of pharmaceutical research : IJPR, 12(2), 331-346 (2013-11-20)
In this work, we reported the synthesis and evaluation of antimycobacterial and antifungal activity of a series of thiocarbohydrazone derivatives which are thiacetazone congeners. The target compounds were synthesized in superior yields by reacting thiocarbohydrazide with different aromatic aldehydes and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service