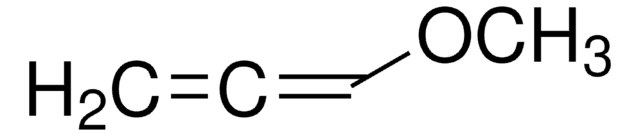212830
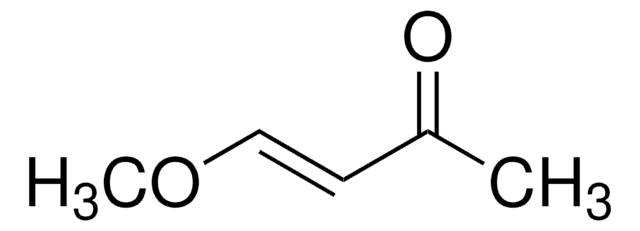
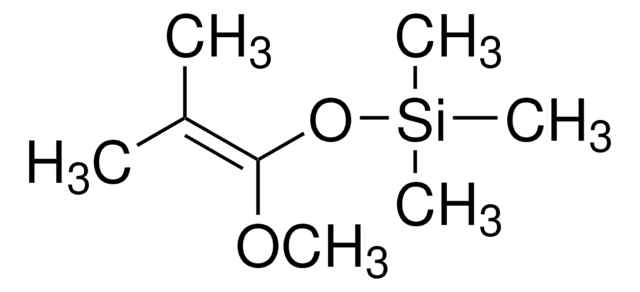
trans-1-Methoxy-3-trimethylsiloxy-1,3-butadiene
95%
Synonym(s):
Danishefsky’s diene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3SiOC(=CH2)CH=CHOCH3
CAS Number:
Molecular Weight:
172.30
Beilstein:
1616761
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
impurities
2-5% 4-methoxy-3-buten-2-one
refractive index
n20/D 1.454 (lit.)
bp
68-69 °C/14 mmHg (lit.)
density
0.885 g/mL at 25 °C (lit.)
functional group
ether
storage temp.
2-8°C
SMILES string
CO\C=C\C(=C)O[Si](C)(C)C
InChI
1S/C8H16O2Si/c1-8(6-7-9-2)10-11(3,4)5/h6-7H,1H2,2-5H3/b7-6+
InChI key
SHALBPKEGDBVKK-VOTSOKGWSA-N
Looking for similar products? Visit Product Comparison Guide
General description
trans-1-methoxy-3-trimethylsiloxy-1,3-butadiene is a funtionalized Diels-Alder diene. Mukaiyama-Michael-type addition/heterocyclization of trans-1-methoxy-3-trimethylsiloxy-1,3-butadiene (Danishefsky′s diene) with 1,2-diaza-1,3-butadiene has been investigated. Asymmetric hetero-Diels-Alder cyclization of Danishefsky′s diene with benzaldehyde catalyzed by mesoporous inorganic/metalorganic hybrid materials has been reported.
Application
trans-1-Methoxy-3-trimethylsiloxy-1,3-butadiene was used:
- in the synthesis of sulfone analogues of griseofulvin (sulfogriseofulvins), 4H-1-aminopyrroles and 4,5H-pyrazoles
- as Diels-Alder diene for the synthesis of pyridones and pyranones
- as reagent employed in the Mannich-Michael reaction for preparation of piperidinones and enaminones
accessory
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
138.2 °F - closed cup
Flash Point(C)
59 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Screening of rare earth metal grafted MCM-41 silica for asymmetric catalysis.
Gerstberger G and Anwander R.
Microporous and Mesoporous Materials : The Official Journal of the International Zeolite Association, 44, 303-310 (2001)
The Journal of Organic Chemistry, 57, 4444-4444 (1992)
Tetrahedron, 49, 397-397 (1993)
Marie-Laure Teyssot et al.
The Journal of organic chemistry, 72(7), 2364-2373 (2007-03-10)
Electron-poor 6-oxo-1-sulfonyl-1,6-dihydropyridine-3-carboxylates 1b-d undergo cross-Diels-Alder reactions with electron-rich dienes 4a-f under hyperbaric conditions, reacting either as dienophiles to yield normal-electron-demand (NED) cycloadducts 10 and/or 11 or as dienes to give inverse-electron-demand (IED) cycloadducts 12 and/or 13. The latter are converted
Orazio A Attanasi et al.
Organic letters, 10(10), 1983-1986 (2008-04-23)
The versatility of the Mukaiyama-Michael-type addition/heterocyclization of Danishefsky's diene with 1,2-diaza-1,3-butadienes was applied to the synthesis of both 4 H-1-aminopyrroles and 4,5 H-pyrazoles. Thus, the same reagents furnished different types of highly functionalized azaheterocycles essentially depending on their structure: as
Articles
Reagents for C–C Bond Formation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service