209392
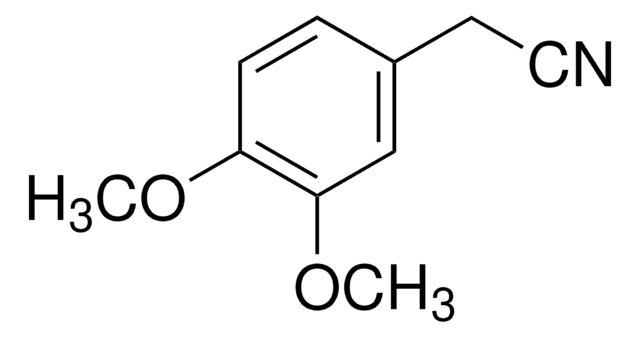
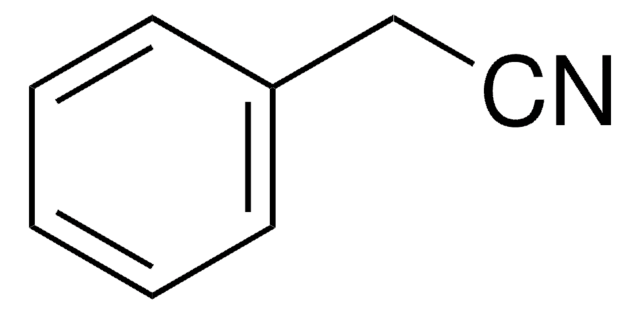
3-Methoxyphenylacetonitrile
99%
Synonym(s):
3-Methoxybenzyl cyanide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3OC6H4CH2CN
CAS Number:
Molecular Weight:
147.17
Beilstein:
1865539
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.532 (lit.)
bp
164-165 °C/20 mmHg (lit.)
density
1.054 g/mL at 25 °C (lit.)
functional group
nitrile
SMILES string
COc1cccc(CC#N)c1
InChI
1S/C9H9NO/c1-11-9-4-2-3-8(7-9)5-6-10/h2-4,7H,5H2,1H3
InChI key
LXKNAUOWEJWGTE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
3-Methoxyphenylacetonitrile was used in the synthesis of:
- new immunogen for homovanillic acid
- β,β′-cyclobisalkylated melatoninergic phenylalkylamides
- α-sec-butyl-3-methoxy phenylacetonitrile, antispasmodic agent
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
208.4 °F - closed cup
Flash Point(C)
98 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Suphannika Intanon et al.
Journal of agricultural and food chemistry, 62(30), 7423-7429 (2014-07-08)
Meadowfoam (Limnanthes alba Hartw. ex Benth.) is an oilseed crop grown in the Willamette Valley of Oregon. Meadowfoam seed meal (MSM), a byproduct after oil extraction, contains 2-4% glucosinolate (glucolimnanthin). Activated MSM, produced by adding freshly ground myrosinase-active meadowfoam seeds
Synthesis of a homovanillic acid immunogen that incorporates an isosteric group designed to generate antibodies with improved specificity.
Gallacher G, et al.
Biogenic Amines, 11(1), 49-62 (1995)
An Efficient Synthesis of Simple, ?,?′-Cyclobisalkylated Melatoninergic Phenylalkylamides.
Tsotinis A, et al.
Letters in Organic Chemistry, 4(2), 92-95 (2007)
Antispasmodic agents. 1. Syntheses and pharmacological activity of aminoalkyl 3-substituted phenylacetates.
T Kametani et al.
Journal of medicinal chemistry, 14(1), 72-75 (1971-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service