206563
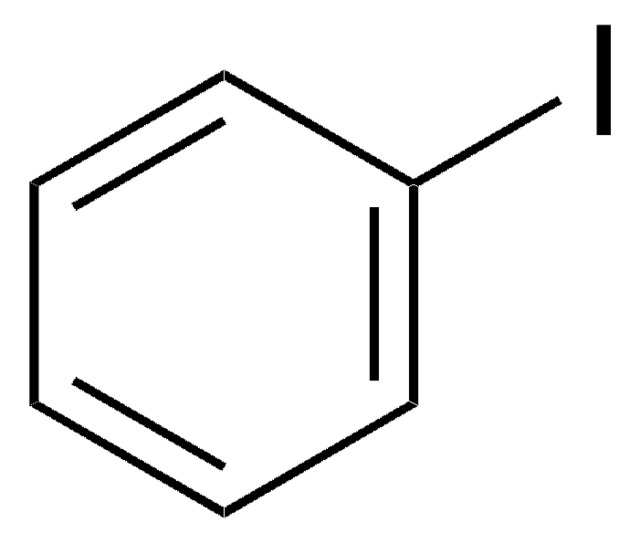
3-Iodobenzotrifluoride
98%
Synonym(s):
3-Iodo-α,α,α-trifluorotoluene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
IC6H4CF3
CAS Number:
Molecular Weight:
272.01
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.517 (lit.)
bp
82-82.5 °C/25 mmHg (lit.)
density
1.887 g/mL at 25 °C (lit.)
SMILES string
FC(F)(F)c1cccc(I)c1
InChI
1S/C7H4F3I/c8-7(9,10)5-2-1-3-6(11)4-5/h1-4H
InChI key
IGISPMBUGPHLBY-UHFFFAOYSA-N
Related Categories
General description
Sonogashira coupling reaction between 3-iodobenzotrifluoride and phenylacetylene using a palladium system has been reported.
Application
3-Iodobenzotrifluoride was used in the preparation of zinc 5,10,15,20-tetrakis(3-(trifluoromethyl)phenylethynyl)porphyrin.
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
158.0 °F - closed cup
Flash Point(C)
70 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rafael Luque et al.
Organic & biomolecular chemistry, 7(8), 1627-1632 (2009-04-04)
An efficient metal-free Sonogashira coupling protocol catalysed by DABCO is reported, where very good conversions and selectivities to the cross-coupling product were obtained under mild reaction conditions. The reported solvent-, phosphane- and metal-free protocol, that uses a cheap base as
Ming-Cheng Kuo et al.
Dalton transactions (Cambridge, England : 2003), (14)(14), 1433-1439 (2007-03-28)
Sonogashira coupling of zinc 5,10,15,20-tetraethynylporphyrin with various phenyl iodides under mild conditions afforded good yields of the corresponding zinc porphyrins. This method is applicable to a variety of aryl iodides including meso-substituted iodoporphyrin to form a conjugated star-shaped multiporphyrin. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service