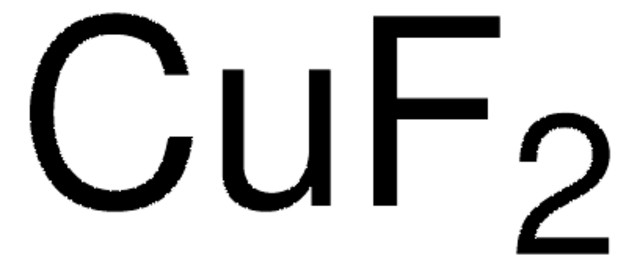All Photos(2)
About This Item
Empirical Formula (Hill Notation):
CsF
CAS Number:
Molecular Weight:
151.90
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.22
grade:
for analytical purposes
form:
liquid
Recommended Products
grade
for analytical purposes
Quality Level
Assay
99%
form
liquid
mp
682 °C (lit.)
density
4.115 g/mL at 25 °C (lit.)
SMILES string
[F-].[Cs+]
InChI
1S/Cs.FH/h;1H/q+1;/p-1
InChI key
XJHCXCQVJFPJIK-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Cesium fluoride is an inorganic compound known to be a source of fluoride ion and a catalyst in organic synthesis. It has been used in many organic reactions like 1,4−elimination, desilylation, transesterification, acylation, nucleophilic aromatic substitution, etherification, cross−coupling reactions and so on.
Application
Cesium fluoride can be used as:
- A base in the Suzuki cross-coupling synthesis of ortho-substituted biaryls.
- A reagent for the nucleophilic fluorination of primary halides and sulfonates in protic media such as tert-butyl and tert-pentyl alcohols.
Reactant for:
- Preparation of building blocks for synthesis of fluoroallylic compounds
- Synthesis of alcohols via hydrolysis of alkyl silyl ethers at neutral pH in buffered mixed organic-aqueous solutions
- Nucleophilic fluorination of alkynyliodonium salts to form fluorovinylic compounds
- Nucleophilic aromatic substitution (SNAr) reactions
Used in the successful synthesis of a single-crystal Dion-Jacobson phase, CsLaTa2O7, that has applications in photocatalysis and superconductivity.
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Repr. 2 - STOT RE 2
Target Organs
Kidney,Adrenal gland
Supplementary Hazards
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
An efficient catalyst for the synthesis of ortho-substituted biaryls by the Suzuki cross-coupling: Triphenylphosphine adduct of cyclopalladated ferrocenylimine
Li H, et al.
Journal of Organometallic Chemistry, 691(26), 5688-5696 (2006)
Cesium Fluoride
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2007)
A cesium fluoride promoted efficient and rapid multicomponent synthesis of functionalized 2-amino-3-cyano-4H-pyran and spirooxindole derivatives
Wagh YB, et al.
Chinese Chemical Letters = Zhongguo Hua Xue Kuai Bao, 26(10), 1273-1277 (2015)
A new class of SN2 reactions catalyzed by protic solvents: facile fluorination for isotopic labeling of diagnostic molecules
Kim DW, et al.
Journal of the American Chemical Society, 128(50), 16394-16397 (2006)
Dong Wook Kim et al.
Journal of the American Chemical Society, 128(50), 16394-16397 (2006-12-15)
Aprotic solvents are usually preferred for the SN2 reactions, because nucleophilicity and hence SN2 reactivity are severely retarded by the influence of the partial positive charge of protic solvents. In this work, we introduce a remarkable effect of using tertiary
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 198323-25G | 4061838762849 |
| 198323-100G | 4061838762832 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service