All Photos(1)
About This Item
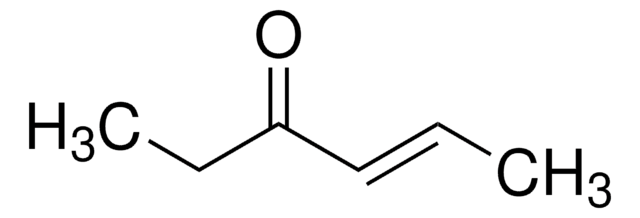
Linear Formula:
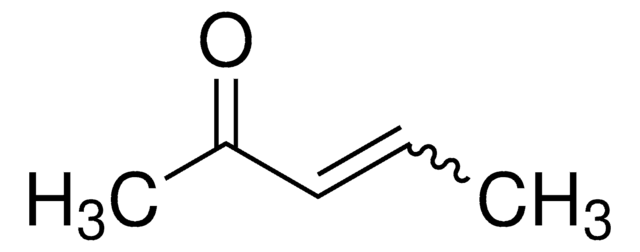
(CH3)2CHCH=CHCOCH3
CAS Number:
Molecular Weight:
112.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
75%
form
liquid
impurities
<25% 5-methyl-4-hexen-2-one
refractive index
n20/D 1.44 (lit.)
density
0.85 g/mL at 25 °C (lit.)
functional group
ketone
SMILES string
CC(C)\C=C\C(C)=O
InChI
1S/C7H12O/c1-6(2)4-5-7(3)8/h4-6H,1-3H3/b5-4+
InChI key
IYMKNYVCXUEFJE-SNAWJCMRSA-N
Related Categories
General description
5-methyl-3-hexen-2-one reacts with indole in the presence of pyrrolidine and p-TsOH in CH2Cl2 to yield the 3-substituted indole adduct.
Application
5-Methyl-3-hexen-2-one was used in combinatorial synthesis of mercaptoketones and mercaptoalcohols.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
118.4 °F - closed cup
Flash Point(C)
48 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
C Vermeulen et al.
Journal of agricultural and food chemistry, 49(11), 5445-5449 (2001-11-21)
Over the past few years, polyfunctional thiols present as trace components have been found to play a major role in many food flavors, due to their exceptionally low odor thresholds. Unfortunately, their presence in minute concentration (in ng/kg to a
Dong-Ping Li et al.
Chemical communications (Cambridge, England), (7)(7), 799-801 (2006-02-09)
The use of an equimolar amount of pyrrolidine and HClO4 (30 mol%) was found to be effective in promoting the conjugate addition of indoles to (E)-alpha,beta-unsaturated ketones, affording the corresponding beta-indolyl ketones in excellent yields.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service