18565
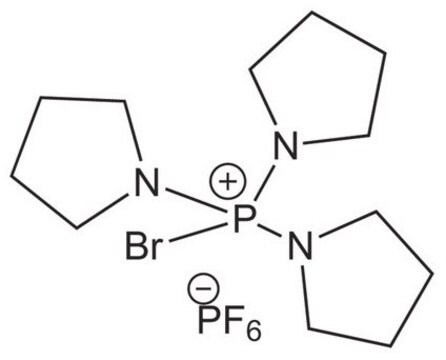
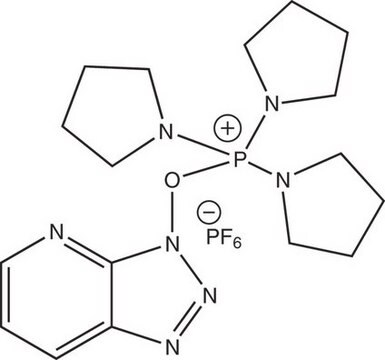
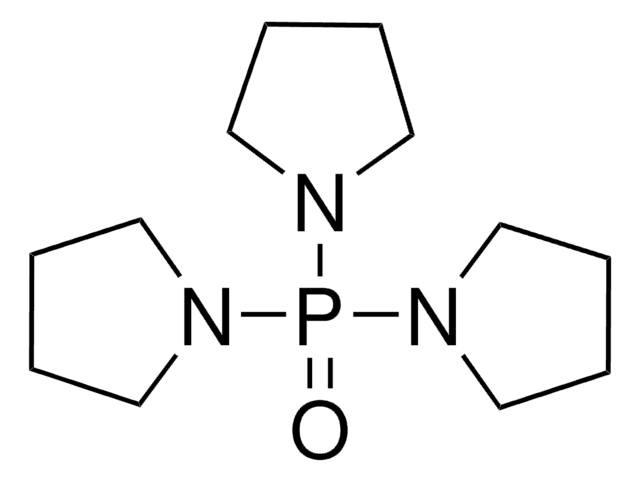
Bromotripyrrolidinophosphonium hexafluorophosphate
≥95.0% (HPLC), for peptide synthesis
Synonym(s):
PyBroP®
About This Item
Recommended Products
product name
Bromotripyrrolidinophosphonium hexafluorophosphate, ≥95.0% (HPLC)
Assay
≥95.0% (HPLC)
form
solid
reaction suitability
reaction type: Coupling Reactions
application(s)
peptide synthesis
storage temp.
−20°C
SMILES string
F[P-](F)(F)(F)(F)F.Br[P+](N1CCCC1)(N2CCCC2)N3CCCC3
InChI
1S/C12H24BrN3P.F6P/c13-17(14-7-1-2-8-14,15-9-3-4-10-15)16-11-5-6-12-16;1-7(2,3,4,5)6/h1-12H2;/q+1;-1
InChI key
CYKRMWNZYOIJCH-UHFFFAOYSA-N
Application
Synthesis of primary amides
Direct dehydrative phosphonium cross coupling
Direct arylation
Pyrrolidide formation by phosphonium salt coupling reagents
- For Suzuki–Miyaura cross-coupling of phenols and arylboronic acids to synthesize biaryls and heterobiaryls.
- To functionalize pyrimidines (synthesized from 3,4-dihydropyrimidin-2(1H)-ones (DHPMs) via the Kappe dehydrogenation) by using various nucleophiles.
- To synthesize formamidines by coupling with various primary amines and N,N-diisopropylethylamine.
It can also be used as an activating reagent:
- For the activation of C-OH bond in tautomerizable heterocycles to form the phosphonium salt which aids the Sonogashira coupling of heterocycles with various alkynes.
- For the one–pot activation of C-O bond in phenols and further coupling reaction with phosphine oxide or phosphite to form C-P bonds.
Other Notes
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service