All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H12O
CAS Number:
Molecular Weight:
160.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.5535 (lit.)
bp
127-131 °C/12 mmHg (lit.)
density
1.057 g/mL at 25 °C (lit.)
functional group
ketone
SMILES string
CC1CCc2ccccc2C1=O
InChI
1S/C11H12O/c1-8-6-7-9-4-2-3-5-10(9)11(8)12/h2-5,8H,6-7H2,1H3
InChI key
GANIBVZSZGNMNB-UHFFFAOYSA-N
Related Categories
General description
2-Methyl-1-tetralone undergoes enantioselective hydrogenation catalyzed by 1,4-diamine-ruthenium(II) complexes.
Application
2-Methyl-1-tetralone was used in enantioselective separation of indan, tetralin and benzosuberan derivatives in the presence of chiral additives by capillary electrophoresis.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Takeshi Ohkuma et al.
Organic letters, 6(16), 2681-2683 (2004-07-30)
A combined system of a RuCl(2)(binap)(1,4-diamine) complex and t-C(4)H(9)OK in i-C(3)H(7)OH catalyzes enantioselective hydrogenation of various 1-tetralone derivatives and some methylated 2-cyclohexenones. Hydrogenation of 2-methyl-1-tetralone under dynamic kinetic resolution gives the cis alcohol with high ee. [reaction: see text]
Chiral separations of indan, tetralin and benzosuberan derivatives by capillary electrophoresis.
Gahm K-H, et al.
Journal of Chromatography A, 793(1), 135-143 (1998)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service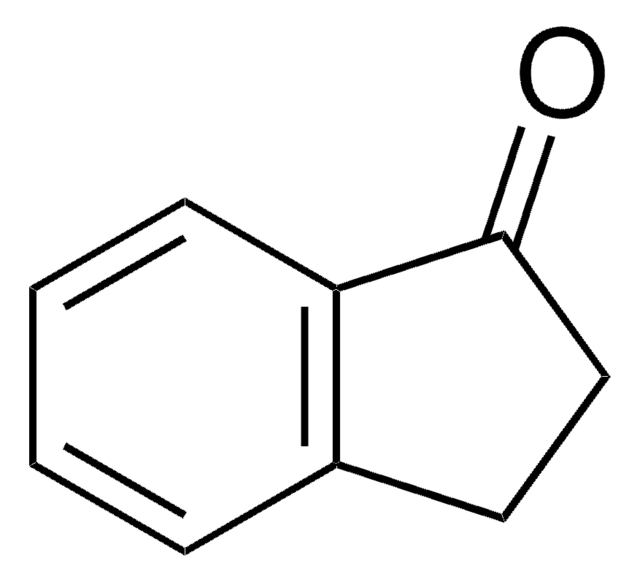
![Bicyclo[2.2.1]hept-2-ene 99%](/deepweb/assets/sigmaaldrich/product/structures/270/492/95fd4909-6108-4858-8c94-609b54387149/640/95fd4909-6108-4858-8c94-609b54387149.png)