158631
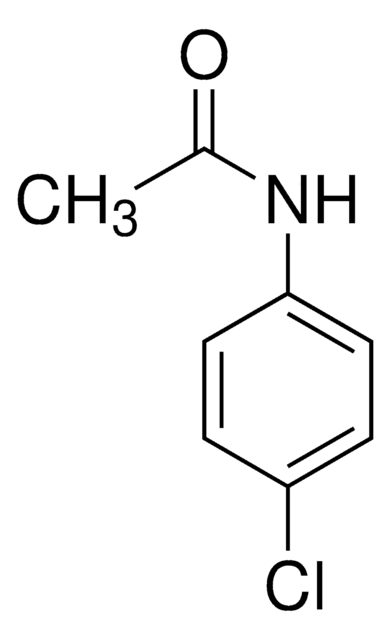
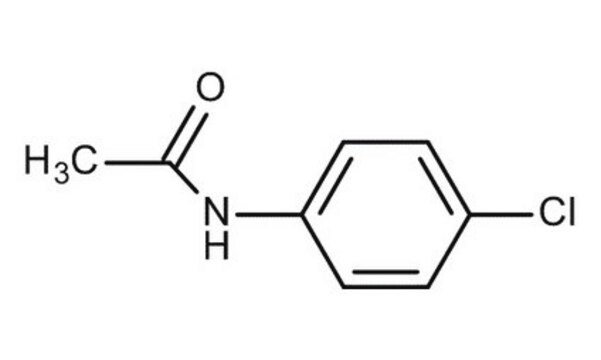
4′-Chloroacetanilide
97%
Synonym(s):
N-(4-Chlorophenyl)acetamide, Acetic acid 4-chloroanilide, NSC 40563, NSC 444
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3CONHC6H4Cl
CAS Number:
Molecular Weight:
169.61
Beilstein:
509638
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
176-178 °C (lit.)
functional group
amide
chloro
SMILES string
CC(=O)Nc1ccc(Cl)cc1
InChI
1S/C8H8ClNO/c1-6(11)10-8-4-2-7(9)3-5-8/h2-5H,1H3,(H,10,11)
InChI key
GGUOCFNAWIODMF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4′-Chloroacetanilide is the main impurity present in acetaminophen and has been quantitated by new high-performance liquid chromatography method using a mixed-mode reversed-phase/cation exchange stationary phase.
Application
<ul>
<li><strong>High-performance liquid chromatography (HPLC) method validation:</strong> 4 chloroacetanilide is used as an internal standard for isosorbide dinitrate in the assay of sustained-release tablets or capsules containing nitroglycerin, isosorbide dinitrate, or pentaerythritol tetranitrate by high-performance liquid chromatography (Gelber and Papas, 1983).</li>
</ul>
<li><strong>High-performance liquid chromatography (HPLC) method validation:</strong> 4 chloroacetanilide is used as an internal standard for isosorbide dinitrate in the assay of sustained-release tablets or capsules containing nitroglycerin, isosorbide dinitrate, or pentaerythritol tetranitrate by high-performance liquid chromatography (Gelber and Papas, 1983).</li>
</ul>
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tamas A Godany et al.
Chimia, 65(4), 253-255 (2011-06-18)
Lithiation of N-(4-chlorophenyl)-pivalamide (NCP) and two additional substituted acetanilides: 4-fluoroacetanilide (4-F) and 4-chloroacetanilide (4-Cl) has been monitored by means of calorimetry, on-line ATR-IR and UV/vis spectroscopy and endoscopy. The combined on-line monitoring revealed the differences between the reaction paths of
L Koymans et al.
Xenobiotica; the fate of foreign compounds in biological systems, 23(6), 633-648 (1993-06-01)
1. The general mechanism of metabolic oxidation of substrates by cytochromes P450 (P450s) appears to consist of sequential one-electron oxidation steps rather than of a single concerted transfer of activated oxygen species from P450 to substrates. 2. In case of
Octavian Călinescu et al.
Journal of chromatographic science, 50(4), 335-342 (2012-03-13)
Determination of acetaminophen and its main impurities: 4-nitrophenol, 4'-chloroacetanilide, as well as 4-aminophenol and its degradation products, p-benzoquinone and hydroquinone has been developed and validated by a new high-performance liquid chromatography method. Chromatographic separation has been obtained on a Hypersil
Jody A Shoemaker et al.
Journal of AOAC International, 89(1), 201-209 (2006-03-04)
U.S. Environmental Protection Agency (EPA) Method 535 has been developed in order to provide a method for the analysis of "Alachlor ESA and other acetanilide degradation products," which are listed on EPA's 1998 Drinking Water Contaminant Candidate List. Method 535
Anthony F Pizon et al.
Clinical toxicology (Philadelphia, Pa.), 47(2), 132-136 (2008-07-09)
p-Chloroaniline is more potent at producing methemoglobin than aniline in animal models. This case highlights the clinical presentation of an inhalation exposure to p-chloroaniline and associated laboratory analysis. An in-vitro study evaluating the metabolism of p-chloroaniline in human hepatocytes was
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 158631-25G | 4061835158133 |
| 158631-100G | |
| 158631-5G | 4061835158140 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service