157872
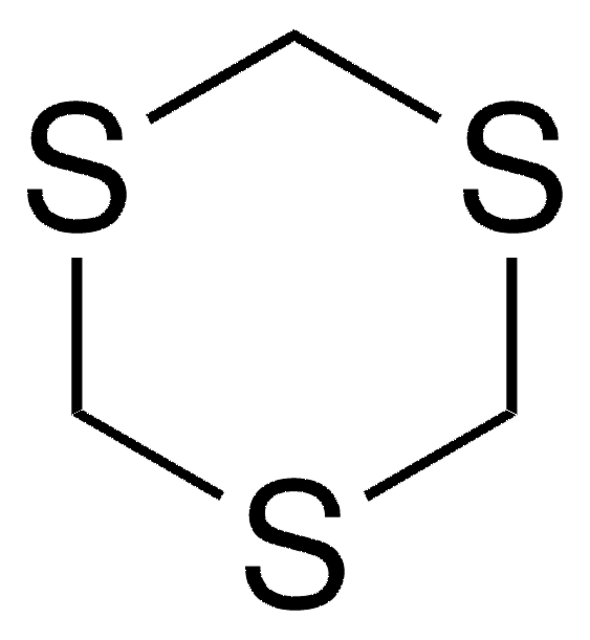
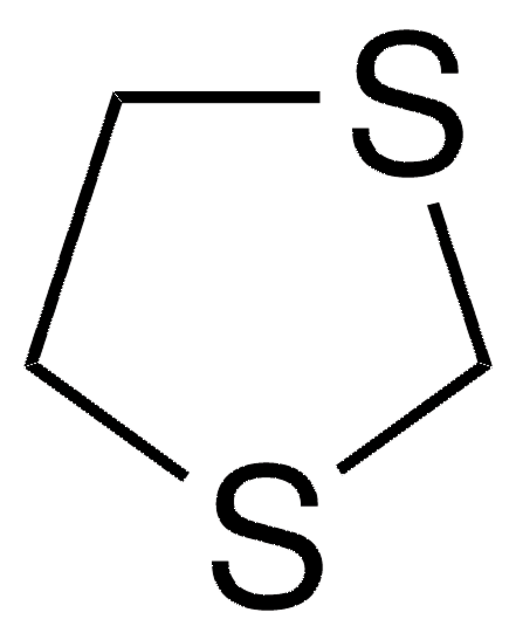
1,3-Dithiane
97%
Synonym(s):
m-Dithiane (7CI), m-Dithiane (8CI)
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4H8S2
CAS Number:
Molecular Weight:
120.24
Beilstein:
102534
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
52-54 °C (lit.)
functional group
thioether
SMILES string
C1CSCSC1
InChI
1S/C4H8S2/c1-2-5-4-6-3-1/h1-4H2
InChI key
WQADWIOXOXRPLN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,3-Dithiane, a protected formaldehyde anion equivalent, serves as useful labeled synthon.
Application
1,3-Dithiane was used as reagent for deoxygenation of sulfoxides to their corresponding sulfides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
194.0 °F - closed cup
Flash Point(C)
90 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A I Noskov et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 77(1), 6-10 (2010-07-17)
The IR spectra of 1,3-dithiane-1-oxide (I) and 1,3-dithia-1-oxocyclohept-5-ene (II) were recorded in solution, solid and liquid phase over 4000-400 cm(-1) spectral range. It was found that both (I) and (II) in liquid phase and solutions exist in two conformations: (I)
Yuncong Chen et al.
Chemical communications (Cambridge, England), 48(42), 5094-5096 (2012-04-20)
A novel sensitive and specific Hg(2+) chemodosimeter, derived from 1',3'-dithiane-substituted 2,1,3-benzoxadiazole, displays "turn-on" fluorescent and colorimetric responses via an Hg(2+)-triggered aldehyde recovery reaction. Its potential to monitor Hg(2+) in living organisms has been demonstrated using zebrafish larvae.
Nasser Iranpoor et al.
The Journal of organic chemistry, 67(9), 2826-2830 (2002-04-27)
A new, mild, and novel method is described for the efficient deoxygenation of sulfoxides to their corresponding sulfides with 1,3-dithiane at room temperature in the presence of catalytic amounts of N-bromosuccinimide (NBS), 2,4,4,6-tetrabromo-2,5-cyclohexadienone (TABCO), or Br(2) as the source of
Al-Monsur Jiaul Haque et al.
Chemical communications (Cambridge, England), (32)(32), 4865-4867 (2009-08-05)
We report the use of 1,3-dithiane combined with aryldiazonium cation for the immobilization of biomolecules based on electrochemical addressing.
Valerie J Peterson et al.
The Biochemical journal, 362(Pt 1), 173-181 (2002-02-07)
Apo and holo forms of retinoic acid receptors, and other nuclear receptors, display differential sensitivity to proteolytic digestion that likely reflects the distinct conformational states of the free and liganded forms of the receptor. We have developed a method for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service