144886
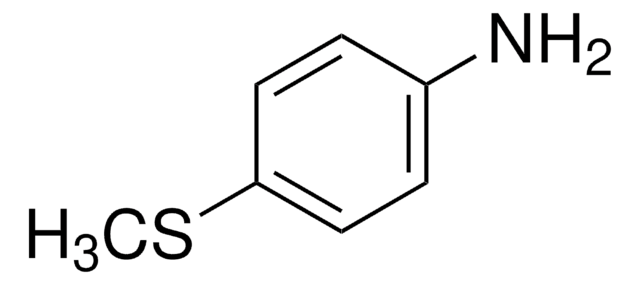
3-(Methylthio)aniline
97%
Synonym(s):
3-(Methylmercapto)aniline, 3-Aminothioanisole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3SC6H4NH2
CAS Number:
Molecular Weight:
139.22
Beilstein:
2078599
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.637 (lit.)
bp
163-165 °C/16 mmHg (lit.)
density
1.13 g/mL at 25 °C (lit.)
functional group
thioether
SMILES string
CSc1cccc(N)c1
InChI
1S/C7H9NS/c1-9-7-4-2-3-6(8)5-7/h2-5H,8H2,1H3
InChI key
KCHLDNLIJVSRPK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
3-(Methylthio)aniline was used in the synthesis of phenyl azobenzene sulfonamide derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Turan Gul et al.
Drug metabolism and disposition: the biological fate of chemicals, 44(8), 1270-1276 (2016-03-18)
Mammalian flavin-containing monooxygenases, which are difficult to obtain and study, play a major role in detoxifying various xenobiotics. To provide alternative biocatalytic tools to generate flavin-containing monooxygenases (FMO)-derived drug metabolites, a collection of microbial flavoprotein monooxygenases, sequence-related to human FMOs
Takashi Ikawa et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 26(19), 4320-4332 (2020-01-03)
Benzynes were selectively generated in situ from phenols and trapped regioselectively with potassium hexamethyldisilazide to form primary anilines following acidic workup. The direct conversion of a phenolic hydroxyl group into a free amino group is a useful method for the
Wei-Jern Tsai et al.
Bioorganic & medicinal chemistry letters, 16(17), 4440-4443 (2006-07-04)
A series of phenylazobenzenesulfonamide derivatives were designed and synthesized for the evaluation as selective cyclooxygenase-2 (COX-2) inhibitors in a cellular assay using human whole blood (HWB) and an enzymatic assay using purified ovine enzymes. Extensive structure-activity relationships (SAR) were studied
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 47772-5G | |
| 47772-1G | 4061826101957 |
| 144886-100G | |
| 144886-5G | 4061837246036 |
| 144886-1G | 4061837246029 |
| 144886-25G |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service