126179
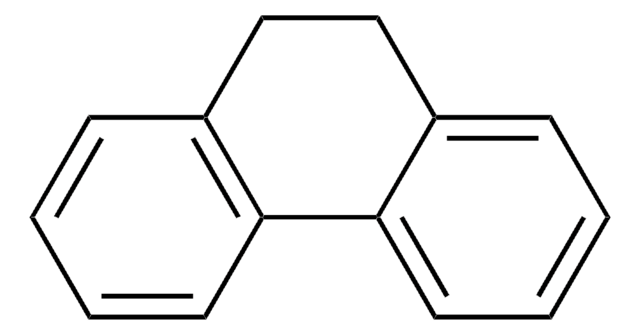
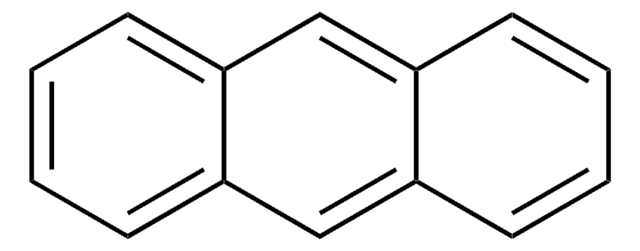
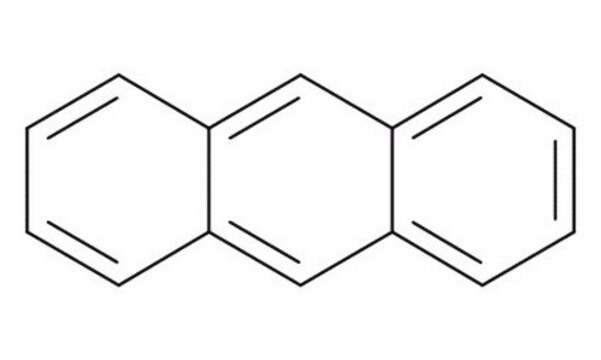
9,10-Dihydroanthracene
97%
Synonym(s):
Dihydroanthracene
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C14H12
CAS Number:
Molecular Weight:
180.25
Beilstein:
1364575
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
bp
312 °C (lit.)
mp
103-107 °C (lit.)
density
0.88 g/mL at 25 °C (lit.)
SMILES string
C1c2ccccc2Cc3ccccc13
InChI
1S/C14H12/c1-2-6-12-10-14-8-4-3-7-13(14)9-11(12)5-1/h1-8H,9-10H2
InChI key
WPDAVTSOEQEGMS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
9,10-Dihydroanthracene causes the transfer hydrogenation of C60 and C70 in the presence of [7H]benzanthrene catalyst. It was oxidatively aromatized to the corresponding anthracene in the presence of molecular oxygen as an oxidant and activated carbon as a promoter in xylene.
Application
9,10-Dihydroanthracene(DHA) has been used in a study to assess the hydrogen abstraction capability of valence-delocalized iron complex with DHA in MeCN.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Genqiang Xue et al.
Proceedings of the National Academy of Sciences of the United States of America, 104(52), 20713-20718 (2007-12-21)
Intermediate Q, the methane-oxidizing species of soluble methane monooxygenase, is proposed to have an [Fe(IV)(2)(mu-O)(2)] diamond core. In an effort to obtain a synthetic precedent for such a core, bulk electrolysis at 900 mV (versus Fc(+/0)) has been performed in
Nirupama Singh et al.
Journal of inorganic biochemistry, 197, 110674-110674 (2019-05-21)
Developing coordination complexes of earth abundant metals that can perform substrate oxidations under benign conditions is an ongoing challenge. Herein, the reactivity of two mononuclear Cu-complexes toward the oxidant H2O2 is reported. Both complexes displayed ligand oxidation upon reaction with
Natsuki Nakamichi et al.
The Journal of organic chemistry, 68(21), 8272-8273 (2003-10-11)
Substituted 9,10-dihydroanthracenes were oxidatively aromatized to the corresponding anthracenes effectively by using molecular oxygen as an oxidant and activated carbon (Darco KB, Aldrich, Inc.) as a promoter in xylene.
[7H] Benzanthrone, a catalyst for the transfer hydrogenation of C60 and C70 by 9, 10-dihydroanthracene.
Gerst M, et al.
Tetrahedron Letters, 34(48), 7729-7732 (1993)
Antoine Burel et al.
Journal of chromatography. A, 1485, 101-119 (2017-01-22)
This study aims at modelling and predicting solute retention in capillary Gas Chromatography (GC) and Flow Modulation comprehensive GC (FM-GCxGC). A new thermodynamic model, taking into account the effects of temperature and pressure, is proposed to describe the variation of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service