SML2569
JH-II-127
≥98% (HPLC)
Synonym(s):
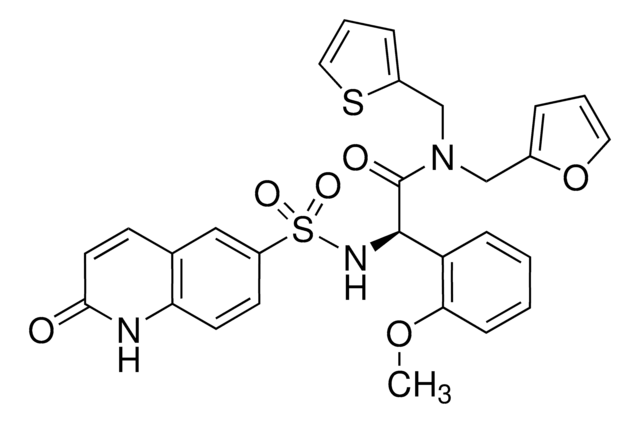
[4-[[5-Chloro-4-(methylamino)-7H-pyrrolo[2,3-d]pyrimidin-2-yl]amino]-3-methoxyphenyl]-4-morpholinylmethanone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C19H21ClN6O3
CAS Number:
Molecular Weight:
416.86
MDL number:
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Assay
≥98% (HPLC)
form
powder
color
white to beige
solubility
DMSO: 2 mg/mL, clear
storage temp.
−20°C
Biochem/physiol Actions
JH-II-127 is an orally available, brain penetrant, potent and selective inhibitor of both wild-type and G2019S mutant Leucine-rich repeat kinase 2 (LRRK2). JH-II-127 inhibits phosphorylation of the Ser910 and Ser935 of both wild-type and G2019S mutant LRRK2. It causes complete inhibition of LRRK2 in mouse brain at 100 mg/kg.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Farzaneh Atashrazm et al.
Clinical pharmacology : advances and applications, 8, 177-189 (2016-11-02)
Major advances in understanding how genetics underlies Parkinson's disease (PD) have provided new opportunities for understanding disease pathogenesis and potential new targets for therapeutic intervention. One such target is leucine-rich repeat kinase 2 (LRRK2), an enigmatic enzyme implicated in both
John M Hatcher et al.
ACS medicinal chemistry letters, 6(5), 584-589 (2015-05-26)
Activating mutations in leucine-rich repeat kinase 2 (LRRK2) are present in a subset of Parkinson's disease (PD) patients and may represent an attractive therapeutic target. Here we report a 2-anilino-4-methylamino-5-chloropyrrolopyrimidine, JH-II-127 (18), as a potent and selective inhibitor of both
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service