All Photos(1)
About This Item
Empirical Formula (Hill Notation):
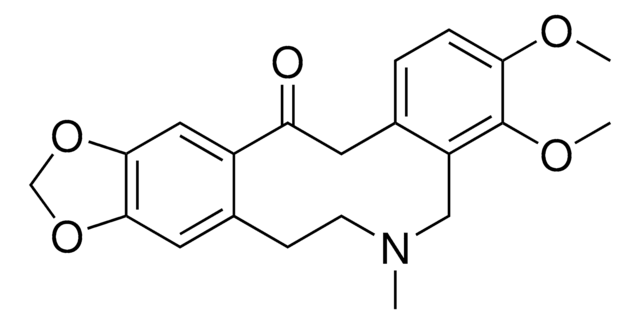
C20H19NO5·HCl
CAS Number:
Molecular Weight:
389.83
EC Number:
MDL number:
UNSPSC Code:
51111800
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98%
form
solid
storage temp.
2-8°C
SMILES string
Cl.CN1CCc2cc3OCOc3cc2C(=O)Cc4ccc5OCOc5c4C1
InChI
1S/C20H19NO5.ClH/c1-21-5-4-13-7-18-19(25-10-24-18)8-14(13)16(22)6-12-2-3-17-20(15(12)9-21)26-11-23-17;/h2-3,7-8H,4-6,9-11H2,1H3;1H
InChI key
NWNVDSJZGYDVQW-UHFFFAOYSA-N
General description
Protopine is a celandine alkaloid and a bioactive compound associated with the plant families including fumariaceae, berberidaceae and papaveraceae. It is metabolized by demethylenation in the presence of the cytochrome enzymes cytochrome P450 family 2 subfamily d polypeptide 1 (CYP2D1) and cytochrome P450 family 2 subfamily c polypeptide 11 (CYP2C11).
Application
Protopine hydrochloride may be used in the calibration curve preparation for the quantification of alkaloids from Fumaria capreolata using liquid chromatography coupled to diode array detection and electrospray ionization tandem mass spectrometry (LC-DAD-MS) and tandem mass spectrometry(MS/MS). It may also be used as an alkaloid in cytotoxicity and permeability studies carcinogenic cell lines.
Biochem/physiol Actions
Protopine possesses anti-parasitic, antimicrobial and anti-inflammatory property. It mediates mitotic arrest by favoring tubulin polymerization. Protopine elicits anti-invasive effects in breast cancer tumor progression.. It also provides protection against oxidative stress-induced cell death.
Protopine hydrochloride is a Ca2+ channel blocker and antiplatelet agent.
Caution
Protect from light.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Deok Sung Bae et al.
BMB reports, 45(2), 108-113 (2012-03-01)
Protopine is an isoquinoline alkaloid contained in plants in northeast Asia. In this study, we investigated whether protopine derived from Hypecoum erectum L could suppress lipopolysaccharide (LPS)-induced inflammatory responses in murine macrophages (Raw 264.7 cells). Protopine was found to reduce
Xiao Wang et al.
Journal of chromatography. A, 1115(1-2), 267-270 (2006-04-20)
pH-zone-refining counter-current chromatography was successfully applied to the separation of alkaloids from a crude extract of Corydalis decumbens (Thunb.) Pers. using a multilayer coil planet centrifuge (CPC). The experiment was performed with a two-phase solvent system composed of methyl tert-butyl
David K Liscombe et al.
The Journal of biological chemistry, 282(20), 14741-14751 (2007-03-29)
S-Adenosyl-l-methionine:tetrahydroprotoberberine cis-N-methyltransferase (EC 2.1.1.122) catalyzes the conversion of (S)-stylopine to the quaternary ammonium alkaloid, (S)-cis-N-methylstylopine, as a key step in the biosynthesis of protopine and benzophenanthridine alkaloids in plants. A full-length cDNA encoding a protein exhibiting 45 and 48% amino
Xianghua Xiao et al.
European journal of pharmacology, 591(1-3), 21-27 (2008-07-08)
Calcium and lipid peroxidation play important roles in oxidative stress-induced cellular injury and apoptosis, which ultimately cause cell death. In this study we examined whether protopine had a neuroprotection against H(2)O(2)-induced injury in PC12 cells. Pretreatment of PC12 cells with
Anshu Rathi et al.
Phytomedicine : international journal of phytotherapy and phytopharmacology, 15(6-7), 470-477 (2008-01-01)
The present investigation demonstrates the hepatoprotective potential of 50% ethanolic water extract of whole plant of Fumaria indica and its three fractions viz., hexane, chloroform and butanol against d-galactosamine induced hepatotoxicity in rats. The hepatoprotection was assessed in terms reduction
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service