P2636
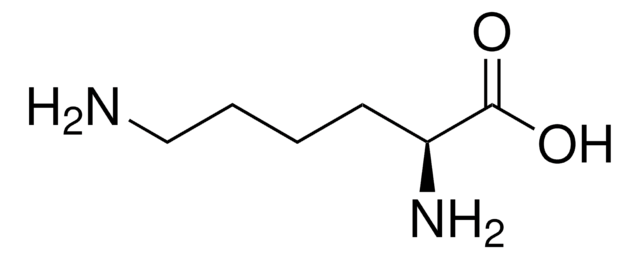
Poly-L-lysine hydrobromide
suitable for cell culture and bioconjugation, Mol wt 30,000-70,000
Synonym(s):
L-Lysine homopolymer hydrobromide
About This Item
Recommended Products
product name
Poly-L-lysine hydrobromide, mol wt 30,000-70,000
form
powder
Quality Level
mol wt
30,000-70,000
technique(s)
bioconjugation: suitable
cell culture | mammalian: suitable
impurities
<10% water (Karl Fischer)
color
white to faint yellow
solubility
water: 50 mg/mL, clear, colorless to light yellow (50 mg/mL, water, Clear, Colorless to light yellow
)
functional group
amine
carboxylic acid
storage temp.
−20°C
SMILES string
Cl.NCCCCC(N)C(O)=O
InChI
1S/C18H38N6O4/c19-10-4-1-7-13(22)16(25)23-14(8-2-5-11-20)17(26)24-15(18(27)28)9-3-6-12-21/h13-15H,1-12,19-22H2,(H,23,25)(H,24,26)(H,27,28)/t13-,14-,15-/m0/s1
InChI key
WBSCNDJQPKSPII-KKUMJFAQSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Derived from the naturally occurring amino acid l-lysine, Poly-L-lysine is available in various forms, allowing for customization in terms of molecular weight and shape. As a synthetic positively charged polymer, it exists in two enantiomers, poly-D-lysine and poly-L-lysine, both of which have their unique applications.
In formulation research, Poly-L-lysine proves to be valuable in nucleic acid delivery. Its positive charge, attributed to the ε-amine on its side chain at physiological pH, enables it to condense plasmid DNA effectively based on salt concentration. While Poly-L-lysine appears suitable for gene delivery, unmodified versions have been associated with low transfection efficiency and cytotoxicity.
In conclusion, Poly-L-lysine offers a multifaceted solution in cell culture and formulation research, enhancing cell adhesion, enabling gene delivery, and contributing significantly to advancements in biochemical and cell biology research.
Application
Biochem/physiol Actions
Features and Benefits
- Can be used in Metabolomics and Biochemical research
- High-quality compound suitable for multiple research applications
Components
Caution
Preparation Note
Analysis Note
Other Notes
comparable product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Kanjiro Miyata (The University of Tokyo, Japan) provides insights on the rational design of polymeric materials for “smart” oligonucleotide delivery.
Humankind has utilized protein materials throughout its existence, starting with the use of materials such as wool and silk for warmth and protection from the elements and continuing with the use of recombinant DNA techniques to synthesize proteins with unique and useful properties.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service