P0108
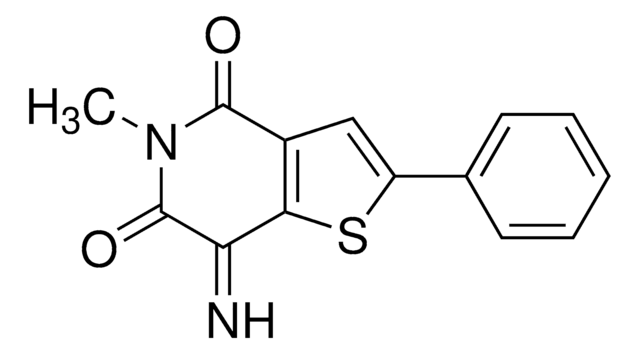
PRL-3 Inhibitor I
≥98% (HPLC), solid
Synonym(s):
5-[[5-Bromo-2-[(2-bromophenyl)methoxy]phenyl]methylene]-2-thioxo-4-thiazolidinone, Phosphatase of regenerating liver-3, Inhibitor I
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
solid
storage condition
protect from light
color
yellow
solubility
DMSO: >10 mg/mL
H2O: <2 mg/mL
storage temp.
−20°C
SMILES string
Brc1ccc(OCc2ccccc2Br)c(c1)\C=C3\SC(=S)NC3=O
InChI
1S/C17H11Br2NO2S2/c18-12-5-6-14(22-9-10-3-1-2-4-13(10)19)11(7-12)8-15-16(21)20-17(23)24-15/h1-8H,9H2,(H,20,21,23)/b15-8+
InChI key
HXNBAOLVPAWYLT-OVCLIPMQSA-N
Application
- to test its effect on classical Hodgkin lymphoma cell survival
- in the human umbilical vein endothelial cells tube formation assay
- to test its effect on the migration of neural crest cells
Biochem/physiol Actions
Features and Benefits
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Protein tyrosine phosphatases (PTPs) and related enzymes (more than a hundred coded by the human genome) are more numerous than serine/threonine phosphatases. They belong to four families, three of which possess a conserved cysteine for catalysis and some conserved features of 3-dimensional structure. The catalytic mechanism of these PTPs involves the transient formation of a covalently phosphorylated enzyme.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service