M9411
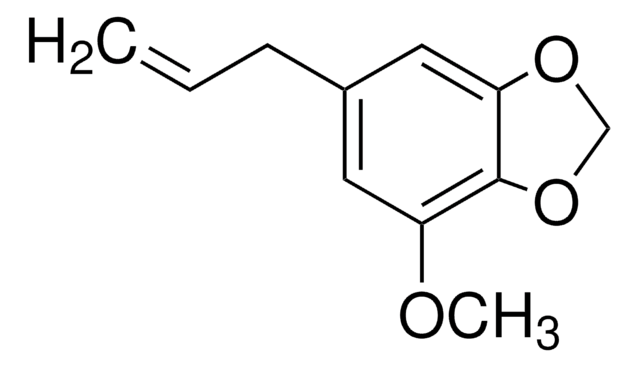
Myristicin from parsley leaf oil
≥85% (HPLC), oil
Synonym(s):
4-Methoxy-6-(2-propenyl)-1,3-benzodioxole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H12O3
CAS Number:
Molecular Weight:
192.21
EC Number:
MDL number:
UNSPSC Code:
12352205
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥85% (HPLC)
form
oil
color
clear light yellow
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
storage temp.
2-8°C
SMILES string
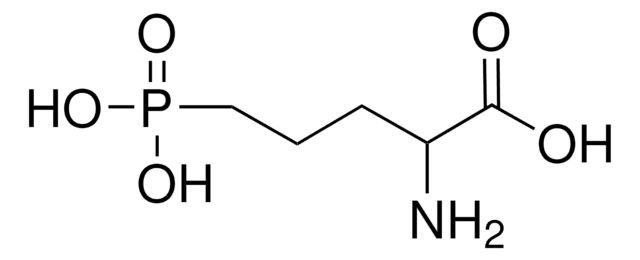
COc1cc(CC=C)cc2OCOc12
InChI
1S/C11H12O3/c1-3-4-8-5-9(12-2)11-10(6-8)13-7-14-11/h3,5-6H,1,4,7H2,2H3
InChI key
BNWJOHGLIBDBOB-UHFFFAOYSA-N
Related Categories
General description
Myristicin, a phenylpropene, is an essential oil component. It has anticholinergic and psychotropic activities. Myristicin blocks cytochrome P450 monooxygenases, which detoxifies furanocoumarins. It functions as a serotonin receptor agonist and hallucinogenic agent. Myristicin stimulates glutathione S-transferase activity and might function as a chemopreventive agent. It acts as a precursor for the metabolite 3,4- methylenedioxymethamphetamine (MDMA).
Application
Myristicin from parsley leaf oil has been used to study competitive and uncompetitive inhibition of ACP (acid phosphatase) and ALP (alkaline phosphatase).
Biochem/physiol Actions
Myristicin induces the expression of glutathione S-transferase and cytochrome P450 (Cyp1a-1) in liver cells. May enhance detoxification of carcinogenic substances.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
STOT SE 3
Target Organs
Central nervous system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
H S Lee et al.
Journal of chromatography. B, Biomedical sciences and applications, 705(2), 367-372 (1998-04-01)
Myristicin [5-allyl-1-methoxy-2,3-(methylenedioxy)benzene] is a flavoring plant constituent and has been known to produce significant psychopharmacological responses as well as insecticidal activity. From in vitro and in vivo metabolism of myristicin, the two metabolites 5-allyl-1-methoxy-2,3-dihydroxybenzene and 1'-hydroxymyristicin were identified using GC-MS
G Q Zheng et al.
Carcinogenesis, 13(10), 1921-1923 (1992-10-01)
Glutathione S-transferase (GST) assay-guided fractionation of parsley leaf oil from the edible plant Petroselinum sativum Hoffm. (Umbelliferae) led to the isolation of myristicin. Myristicin showed high activity as an inducer of the detoxifying enzyme GST in the liver and small
H Ahmad et al.
Biochemical and biophysical research communications, 236(3), 825-828 (1997-07-30)
The present studies were undertaken to elucidate the mechanism of induction of glutathione S-transferase (GST) in mouse liver by myristicin, an active constituent of parsley leaf. A/J albino mice, given 5 to 50 mg doses of myristicin, showed 4- to
Allelochemical induction of cytochrome P450 monooxygenases and amelioration of xenobiotic toxicity in Helicoverpa zea
Zeng RS, et al.
Journal of Chemical Ecology, 33(3), 449-449 (2007)
Myristicin-induced neurotoxicity in human neuroblastoma SK-N-SH cells
Lee BK, et al.
Toxicology Letters, 157(1), 49-56 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service