M4287
Mitomycin C from Streptomyces caespitosus
powder, suitable for cell culture, BioReagent
Synonym(s):
Mitomycin
About This Item
Recommended Products
product name
Mitomycin C from Streptomyces caespitosus, powder, BioReagent, suitable for cell culture
biological source
Streptomyces caespitosus
Quality Level
product line
BioReagent
form
powder
technique(s)
cell culture | mammalian: suitable
color
gray
purple/blue
(1) 3.2, (2) 6.5
solubility
H2O: 4 mL/vial, clear to slightly hazy, blue to purple (Stock solutions should be filter sterilized and stored at 2-8 °C in the dark.)
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
Mode of action
DNA synthesis | interferes
storage temp.
2-8°C
SMILES string
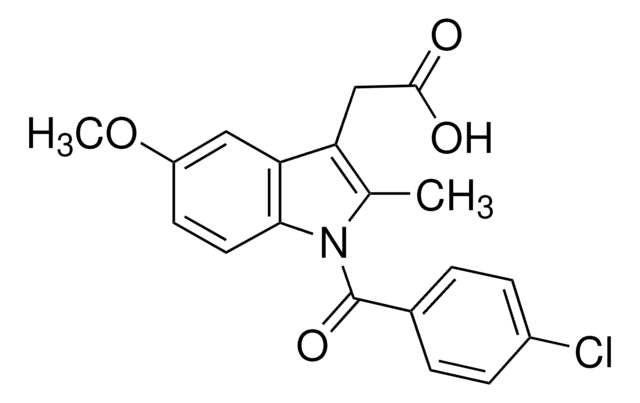
[H][C@]12CN3C4=C([C@@H](COC(N)=O)[C@@]3(OC)[C@@]1([H])N2)C(=O)C(N)=C(C)C4=O
InChI
1S/C15H18N4O5/c1-5-9(16)12(21)8-6(4-24-14(17)22)15(23-2)13-7(18-13)3-19(15)10(8)11(5)20/h6-7,13,18H,3-4,16H2,1-2H3,(H2,17,22)/t6-,7+,13+,15-/m1/s1
InChI key
NWIBSHFKIJFRCO-WUDYKRTCSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Chemical structure: Aziridine
Application
Biochem/physiol Actions
Antimicrobial Spectrum: Mitomycin C has strong antitumor activity, especially against Ehrlich ascites tumor cells, and strong bactericidal action against gram-positive and gram-negative bacteria.
Caution
Preparation Note
related product
suggested gloves for splash protection
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Antibiotic kill curve is a dose response experiment in which mammalian cells are subjected to increasing amounts of selection antibiotic
Protocols
Culturing Trophoblast (TS) Cell Lines Protocol
This technical article covers the indirect co-culture of embryonic stem cells with embryonic fibroblasts.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service