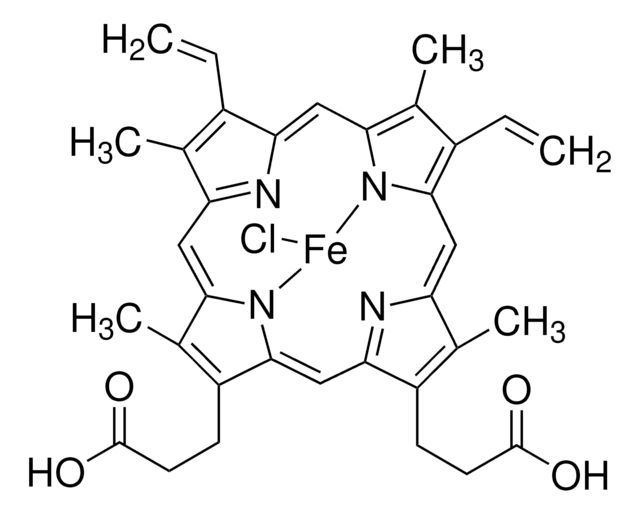
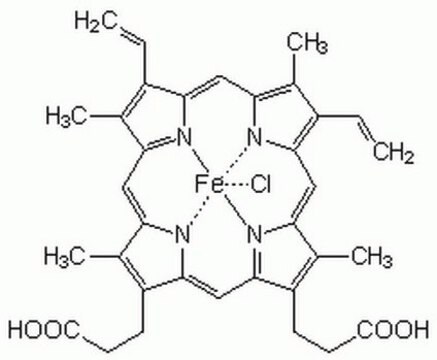
H6390
Hemin−Agarose
Type I, saline suspension
Synonym(s):
Hemin Agarose, Hemin Binding Gel, Hemin-Agarose Gel
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
hemin from bovine
Quality Level
type
Type I
form
saline suspension
extent of labeling
≥4 μmol (per ml)
technique(s)
affinity chromatography: suitable
matrix
Cross-linked 4% beaded agarose
matrix activation
epoxy
matrix attachment
carboxyl
matrix spacer
12 atoms
suitability
suitable for chromatography
storage temp.
2-8°C
Application
Hemin Type I-agarose is an agarose conjugate in saline suspension used in affinity chromatography, protein chromatography and specialty resins. Hemin-agarose has been used to evaluate protection against invasion by Gram-negative bacteria.
Physical form
Suspension in 0.5 M NaCl containing preservative
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Inducible glutathione S-transferase (IrGST1) from the tick Ixodes ricinus is a haem-binding protein
Perner J, et al.
Insect Biochemistry and Molecular Biology, 95, 44-54 (2018)
S Yamamoto et al.
FEMS microbiology letters, 128(2), 195-200 (1995-05-01)
Several clinical isolates of Vibrio parahaemolyticus were examined for their ability to utilize either hemin or hemoglobin as a sole source of iron. Both compounds appeared to be equally good iron sources. Maximum growth was obtained at 5 microM hemin
Hye-Jung Lee et al.
Journal of experimental botany, 63(16), 5967-5978 (2012-09-20)
The Arabidopsis thaliana L. SOUL/haem-binding proteins, AtHBPs belong to a family of five members. The Arabidopsis cytosolic AtHBP1 (At1g17100) and AtHBP2 (At2g37970) have been shown to bind porphyrins and metalloporphyrins including haem. In contrast to the cytosolic localization of these
C S Bracken et al.
Journal of bacteriology, 181(19), 6063-6072 (1999-09-28)
The abilities of two bacterial active heme transporters, HmbR of Neisseria meningitidis and HemR of Yersinia enterocolitica, to use different heme sources were compared. While HmbR-expressing cells used only hemoglobin (Hb) and heme, HemR-expressing bacteria were able to grow on
Swapna Asuthkar et al.
Infection and immunity, 75(9), 4582-4591 (2007-06-20)
In an earlier study, based on the ferric enterobactin receptor FepA of Escherichia coli, we identified and modeled a TonB-dependent outer membrane receptor protein (LB191) from the genome of Leptospira interrogans serovar Lai. Based on in silico analysis, we hypothesized
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service