About This Item
Recommended Products
form
solid
greener alternative product score
old score: 2
new score: 1
Find out more about DOZN™ Scoring
greener alternative product characteristics
Less Hazardous Chemical Syntheses
Designing Safer Chemicals
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
>240 °C (dec.) (lit.)
greener alternative category
storage temp.
room temp
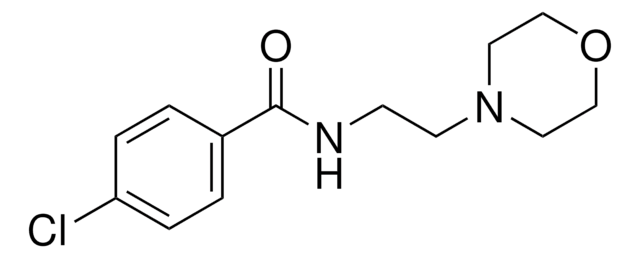
SMILES string
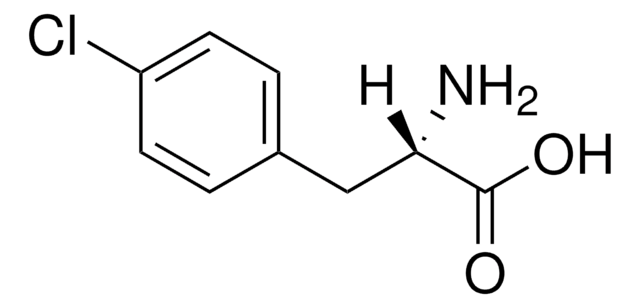
NC(Cc1ccc(Cl)cc1)C(O)=O
InChI
1S/C9H10ClNO2/c10-7-3-1-6(2-4-7)5-8(11)9(12)13/h1-4,8H,5,11H2,(H,12,13)
InChI key
NIGWMJHCCYYCSF-UHFFFAOYSA-N
Gene Information
human ... TPH1(7166) , TPH2(121278)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- as tryptophan hydroxylase 1 (TPH1) inhibitor to treat kras+ male zebrafish
- to induce insomnia in rat models
- used to treat embryos to examine its effect on serotonin
- for the selection of Enterococcus faecalis transformants with pESentA32 plasmid
- to feed flies to explore serotonin effect
Biochem/physiol Actions
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service