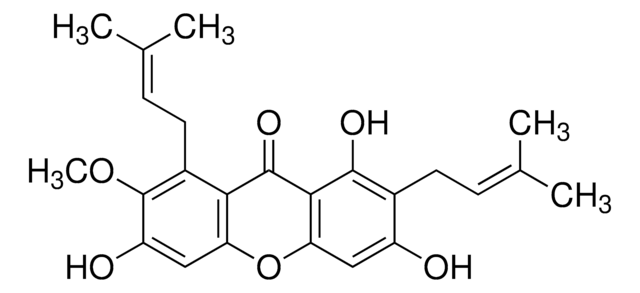
B6938
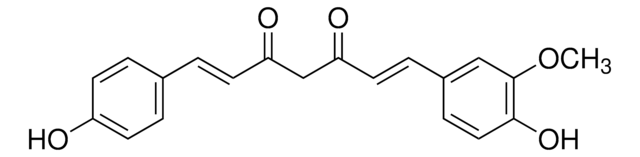
Bisdemethoxycurcumin
≥98% (HPLC), solid
Synonym(s):
(1E,6E)-1,7-bis(4-hydroxyphenyl)hepta-1,6-diene-3,5-dione
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C19H16O4
CAS Number:
Molecular Weight:
308.33
MDL number:
UNSPSC Code:
12352205
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
solid
solubility
DMSO: ≥20 mg/mL
storage temp.
2-8°C
SMILES string
Oc1ccc(cc1)\C=C\C(=O)CC(=O)\C=C\c2ccc(O)cc2
InChI
1S/C19H16O4/c20-16-7-1-14(2-8-16)5-11-18(22)13-19(23)12-6-15-3-9-17(21)10-4-15/h1-12,20-21H,13H2/b11-5+,12-6+
InChI key
PREBVFJICNPEKM-YDWXAUTNSA-N
Application
Bisdemethoxycurcumin has been used:
- to test its inhibitory effect on cell cycle and mitochondrial function in gastric adenocarcinoma cells
- to test it neuroprotective role against lead (Pb) induced toxicity in dopaminergic and noradrenergic systems of Meriones shawi
- as a standard for calibration curve generation to quantify plasma curcuminoids using high-performance liquid chromatography-diode array detection (HPLC-DAD) and ultraviolet (UV)
Biochem/physiol Actions
Bisdemethoxycurcumin (BDMC) is a derivative or curcumin, and represents one of the major active components of curcumin products isolated from Curcumae sp. BDMC shares similar anti-inflammatory properties with demethoxycurcumin. It inhibits LPS-induced nitric oxide (NO) production and expression of iNOS and COX2 in RAW264.7 cells by blocking NF-kB activation. BDMC also displays unique properties in that it enhances Abeta clearance by upregulating expression MGAT3 and TLR genes. BDMC potently inhibits AKR1B10.
Bisdemethoxycurcumin (BMDC) is a stable dimethoxy derivative of curcumin and is useful as a supplement in cell culture medium. It also possesses antimicrobial, antioxidative and neuroprotective functionality.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Lahcen Tamegart et al.
Comptes rendus biologies, 342(5-6), 192-198 (2019-09-03)
Exposure to lead is a threat factor for neurodegenerative disorders progress as it could trigger dopaminergic deficiency. We aimed herein to assess the effect of acute lead exposure (25mg/kg B.W i.p.) during three continuous days on the dopaminergic and noradrenergic
Wojciech Ostrowski
European journal of mass spectrometry (Chichester, England), 21(1), 45-50 (2015-04-24)
Curcumin complexes with iron ions were investigated by electrospray ionization mass spectrometry. It was shown that in methanol solutions of curcumin and iron(III) ions, complexes are formed with a stoichiometry of 1 : 1, 2 : or 3 : 1.
Hannelore Rücker et al.
Organic & biomolecular chemistry, 13(10), 3040-3047 (2015-01-27)
Inflammatory signaling pathways orchestrate the cellular response to infection and injury. These pathways are known to be modulated by compounds that alkylate cysteinyl thiols. One class of phytochemicals with strong thiol alkylating activity is the chalcones. In this study we
Meyliana Wulandari et al.
Analytical and bioanalytical chemistry, 407(3), 803-812 (2014-07-25)
This paper describes the synthesis of novel molecularly imprinted polymers (MIPs), prepared by a noncovalent imprinting approach, for cleanup and preconcentration of curcumin (CUR) and bisdemethoxycurcumin (BDMC) from medicinal herbal extracts and further analysis by high-performance liquid chromatography with fluorescence
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service