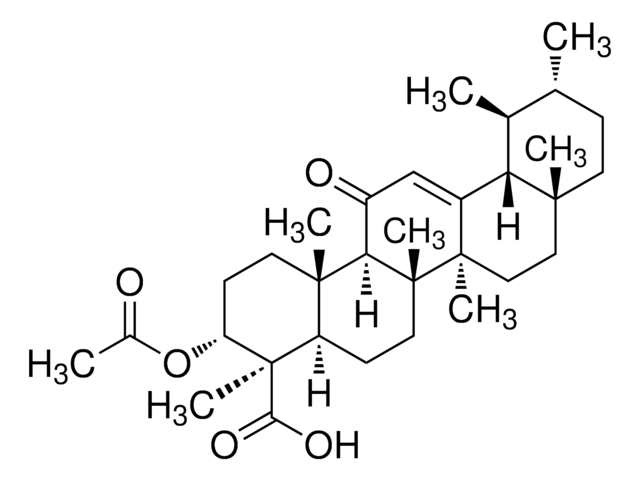
A9855
3-Acetyl-11-keto-β-boswellic acid
from Boswellia serrata
Synonym(s):
AKBA
About This Item
Recommended Products
biological source
Boswellia serrata
form
powder
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
storage temp.
−20°C
InChI
1S/C32H48O5/c1-18-9-12-28(4)15-16-30(6)21(25(28)19(18)2)17-22(34)26-29(5)13-11-24(37-20(3)33)32(8,27(35)36)23(29)10-14-31(26,30)7/h17-19,23-26H,9-16H2,1-8H3,(H,35,36)/t18-,19+,23-,24-,25+,26-,28-,29+,30-,31-,32-/m1/s1
InChI key
HMMGKOVEOFBCAU-BCDBGHSCSA-N
General description
Application
- to test its effects as an anti-proliferating agent on hepatic stellate cells (HSCs) proliferation and synergism in combination with imatinib in in vitro models
- to study its anti-osteoporotic activity on ovariectomy-induced osteoporosis in female Sprague Dawley rats
- in a comparative study with aspirin to test its effects as an anti-inflammatory drug to prevent intestinal adenomatous polyposis in APCMin/+ mice
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service