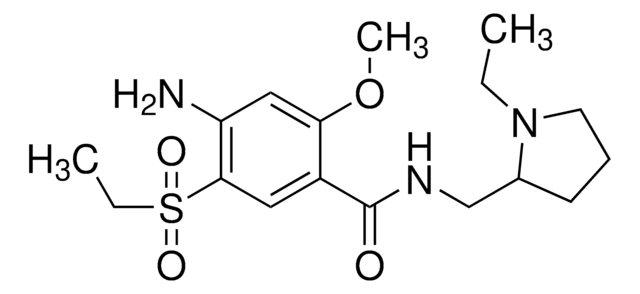
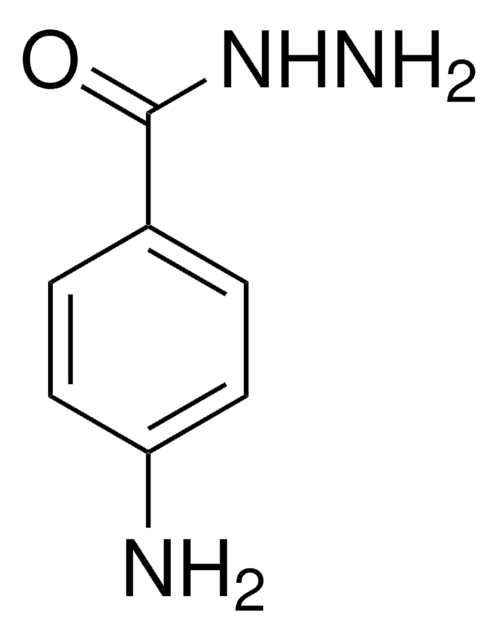
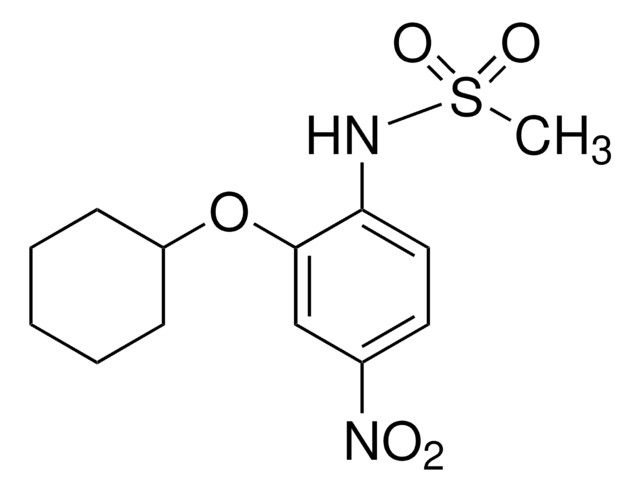
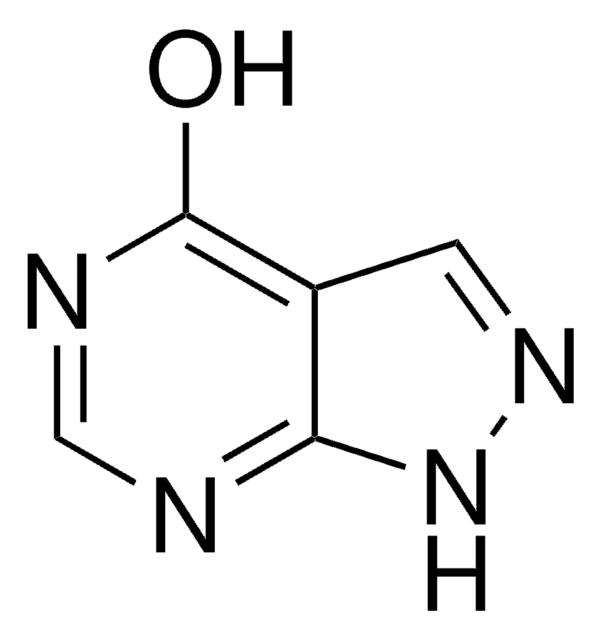
A5170
Anti-Avidin antibody produced in rabbit
whole antiserum
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
rabbit
Quality Level
conjugate
unconjugated
antibody form
whole antiserum
antibody product type
primary antibodies
clone
polyclonal
contains
15 mM sodium azide
technique(s)
immunoelectrophoresis: suitable
indirect ELISA: 1:100,000
shipped in
dry ice
storage temp.
−20°C
target post-translational modification
unmodified
General description
Avidin is a homotetrameric glycoprotein found in the egg white of birds, reptiles and amphibians. Each subunit is 16 kDa, singly glycosylated and binds to a molecule of biotin with greater affinity and specificity. Recombinant avidin from corn is similar to avidin from egg white in terms of properties like isoelectric point (pI) and antigenic properties. Avidin from corn has low molecular weight than chicken egg-derived avidin. Commercial production of avidin from corn has certain advantages in terms of availability of greater biomass and avoiding the co-purification of animal virus.
Immunogen
avidin
Application
Anti-Avidin antibody produced in rabbit has been used in enzyme-linked immunosorbent assay (ELISA). It has also been used in preparation of antibody-Au nanoparticles.
The presence of Scavidin in BT4C glioma cells was assessed by western blot using a rabbit anti-avidin antibody as the primary at a dilution of 1:4000. Lehtolainen, P. (2002) Cloning and Characterization of Scavidin, a Fusion Protein for the Targeted Delivery of Biotinylated Molecules. J. Biol. Chem. 277:8545-8550.
Biochem/physiol Actions
Avidin binds strongly to biotin. Thus, avidin-biotin association has been utilized in immunoassays to detect the localization of antigens in tissues. The use of avidin-biotin immunoassay enhances the sensitivity of the technique and facilitates the detection of antigens in low quantities.
Physical form
Supplied as a liquid containing 15mM sodium azide as preservative
Preparation Note
treated to remove lipoproteins
Analysis Note
This antisera is evaluated for performance and specificity by immunodiffusion and immunoelectrophoresis.
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
10 - Combustible liquids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Coomassie Brilliant Dyes as Surface-Enhanced Raman Scattering Probes for Protein-Ligand Recognitions
Han, Xiao X. and others
Analytical Chemistry, 82(10), 4102-4106 (2010)
Commercial plant-produced recombinant avidin
Commercial Plant-Produced Recombinant Protein Products, 15-25 (2014)
Development of transgenic wheat (Triticum aestivum L.) expressing avidin gene conferring resistance to stored product insects
Abouseadaa HH, et al.
BMC plant biology, 15(1), 1-8 (2015)
Xiao X Han et al.
Analytical chemistry, 82(10), 4102-4106 (2010-04-24)
Coomassie brilliant dyes have high affinity to proteins and high Raman activity, and on the basis of which, we have employed brilliant blue R-250 (BBR) and brilliant blue G-250 (BBG) as surface-enhanced Raman scattering (SERS) labels to probe protein-ligand recognitions.
Colleen Murray et al.
Transgenic research, 19(6), 1041-1051 (2010-03-11)
The high affinity biotin-binding proteins (BBPs) avidin and streptavidin are established insecticidal agents, effective against a range of insect pests. Earlier work showed that, when expressed in planta, full length avidin and a truncated form of streptavidin are highly insecticidal.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service