A2795
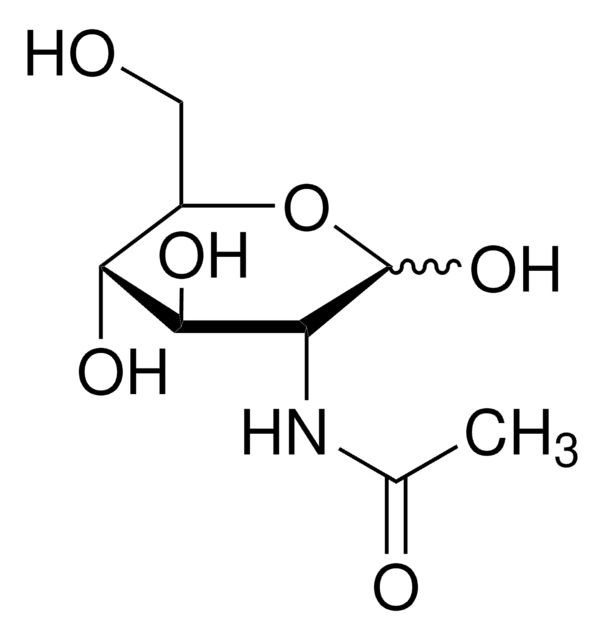
N-Acetyl-D-galactosamine
≥98% (HPLC)
Synonym(s):
2-Acetamido-2-deoxy-D-galactose, D-GalNAc, N-Acetylchondrosamine
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
technique(s)
HPLC: suitable
color
white
mp
160 °C
solubility
water: 50 mg/mL, clear, colorless
storage temp.
2-8°C
SMILES string
CC(=O)N[C@H]1C(O)O[C@H](CO)[C@H](O)[C@@H]1O
InChI
1S/C8H15NO6/c1-3(11)9-5-7(13)6(12)4(2-10)15-8(5)14/h4-8,10,12-14H,2H2,1H3,(H,9,11)/t4-,5-,6+,7-,8?/m1/s1
InChI key
OVRNDRQMDRJTHS-KEWYIRBNSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- as a Dolichos biflorus agglutinin (DBA) haptenic sugar in lectin bead binding assay
- to assess the specificity of lectin binding using lectin blot inhibition
- in immunohistochemistry to pre-adsorb Wisteria floribunda lectin
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service