M0375
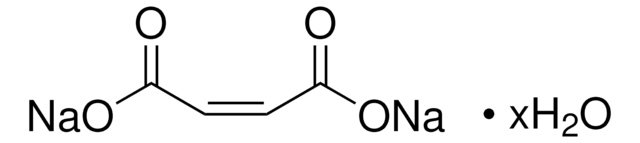
Maleic acid
ReagentPlus®, ≥99% (HPLC)
Synonym(s):
cis-Butenedioic acid, Toxilic acid
About This Item
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
≥99% (HPLC)
form
powder
impurities
≤2.0% water
ign. residue
≤0.1%
pKa (25 °C)
(1) 1.97, (2) 6.24
mp
130-135 °C (lit.)
density
1.59 g/mL at 25 °C (lit.)
functional group
carboxylic acid
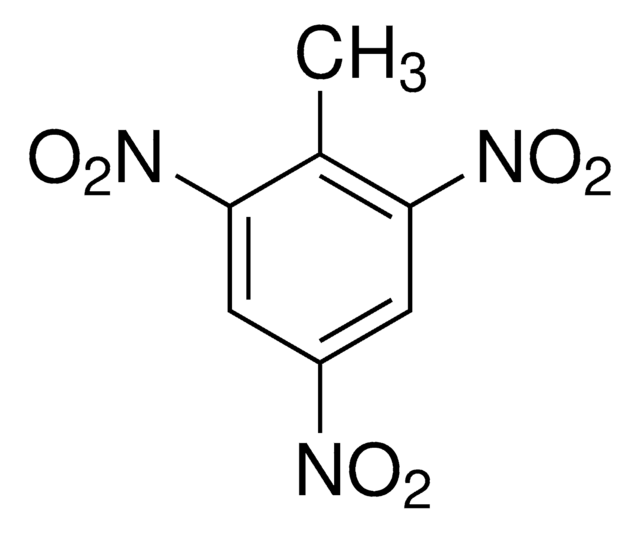
SMILES string
OC(=O)\C=C/C(O)=O
InChI
1S/C4H4O4/c5-3(6)1-2-4(7)8/h1-2H,(H,5,6)(H,7,8)/b2-1-
InChI key
VZCYOOQTPOCHFL-UPHRSURJSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Quality
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
In Situ Hybridization of Whole-Mount Mouse Embryos with RNA Probes: Hybridization, Washes, and Histochemistry. This is a protocol describing how to perform in situ hybridization on whole mouse embryos. Here we describe the hybridization procedure, and the localization of the DIG-labeled RNA using a conjugate of anti-DIG Fab antibody and calf intestinal alkaline phosphatase. Enzyme activity of the reporter is detected by a color reaction, resulting in the formation of a water-insoluble purple/blue precipitate. Manipulating the Mouse Embryo - Third Edition
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service