80126
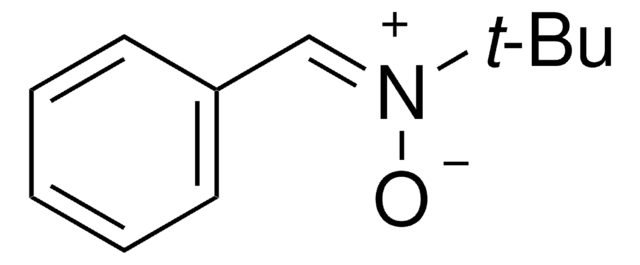
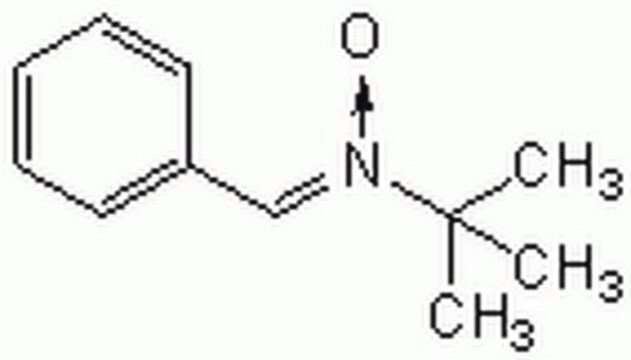
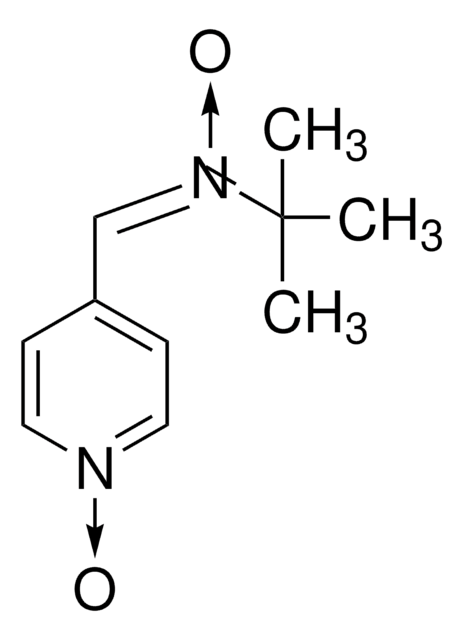
N-tert-Butyl-α-phenylnitrone
for ESR-spectroscopy
Synonym(s):
N-Benzylidene-tert-butylamine N-oxide, PBN, Phenyl N-t-butylnitrone
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH=N(O)C(CH3)3
CAS Number:
Molecular Weight:
177.24
Beilstein:
2044028
EC Number:
MDL number:
UNSPSC Code:
12000000
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
grade
for ESR-spectroscopy
Quality Level
Assay
≥99.5% (HPLC)
form
powder
mp
72-74 °C
73-74 °C (lit.)
solubility
chloroform: 50 mg/mL, clear, colorless
storage temp.
−20°C
SMILES string
CC(C)(C)[N+](\[O-])=C\c1ccccc1
InChI
1S/C11H15NO/c1-11(2,3)12(13)9-10-7-5-4-6-8-10/h4-9H,1-3H3/b12-9-
InChI key
IYSYLWYGCWTJSG-XFXZXTDPSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N-tert-Butyl-a-phenylnitrone was used as spin trapping agent during measurement of scavenging rate constant of carotenoid using EPR spin-trapping technique. This reagent helps in obtaining a six-line ESR spectrum and hyperfine coupling constants, confirming the presence of carbon-based radical in uric acid and peroxynitrite, using electron spin resonance spectroscopy and liquid chromatography mass spectrometry.
Biochem/physiol Actions
N-tert-butyl-α-phenylnitrone (PBN) is a commonly used free-radical spin trap.
N-tert-butyl-α-phenylnitrone (PBN) is a commonly used free-radical spin trap. It has been shown to reduce the number of emboli-induced cerebral microinfarctions in the rabbit cortex and prevent neoplasia by its radical scavenging activity and its ability to inhibit cyclooxygenase-2 activity. Reported to inhibit the induction of nitric oxide synthase (iNOS), thereby preventing the overproduction of nitric oxide (NO). PBN in a dose of 100 mg/kg i.p. reduced necrosis of the substantia nigra, pars reticulate in flurothyl-induced status epilepticus in rats. It protects against some types of post-trauma epileptogenic events in an animal model of epilepsy. The lethal dose of PBN in rats was found to be approximately 100 mg/100 g body weight (0.564 mmol/100Å g).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Antioxidant and redox properties of supramolecular complexes of carotenoids with beta-glycyrrhizic acid.
Polyakov NE
Free Radical Biology & Medicine, 40(10), 1804-1809 (2006)
Radicals in the reaction between peroxynitrite and uric acid identified by electron spin resonance spectroscopy and liquid chromatography mass spectrometry.
Imaram W
Free Radical Biology & Medicine, 49(2), 275-281 (2010)
Mark C Fogarty et al.
Environmental and molecular mutagenesis, 52(1), 35-42 (2010-09-15)
Exercise-induced deoxyribonucleic acid (DNA) damage is often associated with an increase in free radicals; however, there is a lack of evidence examining the two in parallel. This study tested the hypothesis that high-intensity exercise has the ability to produce free
Zhen Wang et al.
Toxicology and applied pharmacology, 259(2), 236-247 (2012-01-19)
The placenta is essential for sustaining the growth of the fetus. An increased endoplasmic reticulum (ER) stress has been associated with the impaired placental and fetal development. Cadmium (Cd) is a potent teratogen that caused fetal malformation and growth restriction.
Ritesh P Daya et al.
Brain research, 1412, 28-36 (2011-08-06)
Haloperidol (HP) is a widely prescribed antipsychotic drug used for the treatment of mental disorders. However, while providing therapeutic benefits, this drug also causes serious extrapyramidal side effects, such as tardive dyskinesia (TD). Upon chronic administration, HP causes behavioural supersensitivity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service