20506
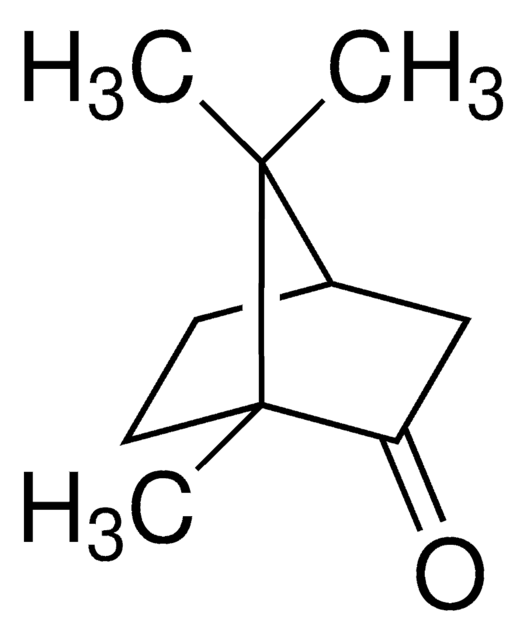
(±)-Camphor
meets analytical specification of Ph. Eur., BP, ≥95% (GC), racemic
Synonym(s):
1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one
About This Item
Recommended Products
vapor density
5.2 (vs air)
Quality Level
vapor pressure
4 mmHg ( 70 °C)
Assay
≥95% (GC)
optical activity
[α]20/D +0.15 to -0.15°, c = 10% in ethanol
quality
meets analytical specification of Ph. Eur., BP
racemic
expl. lim.
3.5 %
impurities
acidity or alcalinity, complies
related subst., complies (GC)
residual solvents, complies
water, complies
≤0.01% halogene compounds (as Cl)
≤0.05% non-volatile matter
bp
204 °C (lit.)
mp
172-180 °C
175-177 °C (lit.)
solubility
carbon disulfide: freely soluble
hexane: freely soluble
liquid sulfur dioxide: soluble
phenol/1,2-dichlorobenzene: soluble
suitability
complies for appearance of solution
passes test for identity
application(s)
pharmaceutical (small molecule)
SMILES string
[H][C@](CC1=O)(CC2)C(C)(C)[C@]12C
InChI
1S/C10H16O/c1-9(2)7-4-5-10(9,3)8(11)6-7/h7H,4-6H2,1-3H3/t7-,10+/m1/s1
InChI key
DSSYKIVIOFKYAU-XCBNKYQSSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Aquatic Chronic 2 - Eye Dam. 1 - Flam. Sol. 2 - Skin Irrit. 2 - STOT SE 2 Inhalation
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 1
Flash Point(F)
147.9 °F - closed cup
Flash Point(C)
64.4 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
-3,7-Dimethyl-2,6-octadien-1-ol; Neral; Geraniol; Geranial; Undecanal; Citronellyl acetate; Neryl acetate; 3,7-Dimethyl-2,6-octadienyl acetate; 1-Tetradecene; Tetradecane; α-Bisabolol
Chromatograms
suitable for GCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service